Cambrian Geology and Paleontology/Volume 2/Middle Cambrian Branchiopoda, Malacostraca, Trilobita, and Merostomata
SMITHSONIAN MISCELLANEOUS COLLECTIONS
VOLUME 57, NUMBER 6
CAMBRIAN
GEOLOGY AND PALEONTOLOGY
II
No. 6.—MIDDLE CAMBRIAN BRANCHIOPODA,
MALACOSTRACA, TRILOBITA, AND
MEROSTOMATA
With Plates 24 to 34
BY
CHARLES D. WALCOTT
(Publication 2051)
CITY OF WASHINGTON
PUBLISHED BY THE SMITHSONIAN INSTITUTION
MARCH 13, 1912
The Lord Baltimore Press
BALTIMORE, MD., U. S. A.
CAMBRIAN GEOLOGY AND PALEONTOLOGY
II
No. 6.—MIDDLE CAMBRIAN BRANCHIOPODA,
MALACOSTRACA, TRILOBITA, AND
MEROSTOMATA
By CHARLES D. WALCOTT
(With Plates 24 to 34)
CONTENTS
| PAGE | ||||
| Introduction | 148 | |||
| Habitat | 149 | |||
| Character of the shale | 149 | |||
| Mode of occurrence | 151 | |||
| Classification | 153 | |||
| Stratigraphic distribution | 155 | |||
| Structural features | 157 | |||
| Exoskeleton | 157 | |||
| Labrum | 158 | |||
| Segmentation | 158 | |||
| Appendages | 158 | |||
| Alimentary canal | 160 | |||
| Hepatic cæca | 160 | |||
| Origin of Middle Cambrian crustacean fauna | 160 | |||
| Relation to recent crustaceans | 164 | |||
| Survival of the Branchiopoda | 165 | |||
| Class Crustacea | 166 | |||
| Sub-Class Branchiopoda | 166 | |||
| Order Anostraca Calman | 166 | |||
| Family Opabinidæ, new family | 166 | |||
| Genus Opabinia, new genus | 166 | |||
| Opabinia regalis, new species (pls. 27, 28.) | 167 | |||
| Appendages | 167 | |||
| Interior structure | 168 | |||
| Dimensions | 168 | |||
| Female | 169 | |||
| Opabinia ? media, new species | 170 | |||
| Genus Leanchoilia, new genus | 170 | |||
| PAGE | ||||
| Leanchoilia superlata, new species (pl. 31) | 170 | |||
| Genus Yohoia, new genus | 171 | |||
| Yohoia tenuis, new species (pl. 29) | 172 | |||
| Appendages | 172 | |||
| Dimensions | 173 | |||
| Yohoia plena, new species (pl. 29) | 173 | |||
| Genus Bidentia, new genus | 173 | |||
| Bidentia difficilis, new species (pl. 30) | 174 | |||
| Appendages | 174 | |||
| Dimensions | 174 | |||
| Order Notostraca | 175 | |||
| Family Naraoidæ, new family | 175 | |||
| Genus Naraoia, new genus | 175 | |||
| Naraoia compacta, new species (pl. 28) | 175 | |||
| Appendages | 176 | |||
| Interior structure | 176 | |||
| Family Burgessidæ, new family | 177 | |||
| Genus Burgessia, new genus | 177 | |||
| Burgessia bella, new species (pls. 27, 30) | 177 | |||
| Eyes | 178 | |||
| Labrum | 178 | |||
| Appendages | 178 | |||
| Interior structure | 179 | |||
| Dimensions | 179 | |||
| Family (Undetermined) | 180 | |||
| Genus Anomalocaris Whiteaves | 180 | |||
| Anomalocaris gigantea, new species (pl. 34) | 180 | |||
| Family Waptidæ, new family | 180 | |||
| Genus Waptia, new genus | 181 | |||
| Waptia fieldensis, new species (pl. 27) | 181 | |||
| Appendages | 182 | |||
| Alimentary canal | 182 | |||
| Dimensions | 182 | |||
| Sub-Class Malacostraca | 182 | |||
| Order Hymenocarina Clarke | 182 | |||
| Family Hymenocaridæ Salter | 182 | |||
| Genus Hymenocaris Salter | 182 | |||
| Hymenocaris perfecta, new species (pl. 31) | 183 | |||
| Appendages | 184 | |||
| Interior structure | 184 | |||
| Dimensions | 184 | |||
| Hymenocaris ? circularis, new species (pl. 32) | 184 | |||
| Hymenocaris obliqua, new species (pl. 32) | 185 | |||
| Hymenocaris ovalis, new species (pl. 32) | 185 | |||
| Hymenocaris ? parva, new species (pl. 32) | 185 | |||
| Family (Undetermined) | 186 | |||
| Genus Hurdia, new genus | 186 | |||
| Hurdia victoria, new species (pl. 32) | 186 | |||
| Hurdia triangulata, new species (pl. 34) | 186 | |||
| Genus Tuzoia, new genus | 187 | |||
| Tuzoia retifera, new species (pl. 33) | 187 | |||
| PAGE | ||||
| Genus Odaraia, new genus | 187 | |||
| Odaraia alata, new species | 188 | |||
| Genus Fieldia, new genus | 188 | |||
| Fieldia lanceolata, new species (pl. 32) | 188 | |||
| Genus Carnarvonia, new genus | 189 | |||
| Cornarvonia venosa, new species (pl. 33) | 189 | |||
| Sub-Class Trilobita | 190 | |||
| Notes on appendages of Neolenus and Ptychoparia (pl. 24) | 191, 192 | |||
| Descriptions of new genera and species | 192 | |||
| Order (Undetermined) | 192 | |||
| Family Marrellidæ, new family | 192 | |||
| Genus Marrella, new genus | 192 | |||
| Marrella splendens, new species (pls. 25, 26) | 193 | |||
| Family (Undetermined) | 194 | |||
| Genus Nathorstia, new genus | 194 | |||
| Nathorstia transitans, new species (pl. 28) | 194 | |||
| Appendages | 195 | |||
| Dimensions | 195 | |||
| Order Hypoparia Beecher | 195 | |||
| Family (Undetermined) | 195 | |||
| Genus Mollisonia, new genus | 196 | |||
| Mollisonia symmetrica, new species (pl. 24) | 196 | |||
| Mollisonia gracilis, new species (pl. 24) | 197 | |||
| Mollisonia ? rara, new species (pl. 24) | 198 | |||
| Genus Tontoia, new genus | 198 | |||
| Tontoia kwaguntensis, new species (pl. 24) | 199 | |||
| Sub-Class Merostomata | 199 | |||
| Order Aglaspina, new order | 199 | |||
| Family Aglaspidæ Clarke | 200 | |||
| Genus Molaria, new genus | 200 | |||
| Molaria spinifera, new species (pl. 29) | 200 | |||
| Appendages | 200 | |||
| Interior structure | 201 | |||
| Dimensions | 201 | |||
| Genus Habelia, new genus | 201 | |||
| Habelia optata, new species (pl. 29) | 202 | |||
| Appendages | 202 | |||
| Surface | 202 | |||
| Dimensions | 203 | |||
| Genus Emeraldella, new genus | 203 | |||
| Emeraldella brocki, new species (text fig. 8 and pl. 30) | 203 | |||
| Appendages | 203 | |||
| Alimentary canal | 204 | |||
| Dimensions | 204 | |||
| Emeraldella micrura, new species (text fig. 9) | 205 | |||
| Order Limulava Walcott | 205 | |||
| Family Sidneyidæ Walcott | 205 | |||
| Genus Sidneyia Walcott | 205 | |||
| Sidneyia inexpectans Walcott (text fig. 10) | 205 | |||
PLATES
| PAGE | |||
| Plate | 24. | Neolenus serratus, Ptychoparia cordilleræ, Mollisonia symmetrica, Tontoia kwaguntensis, Mollisonia gracilis, and Mollisonia ? rara | 208 |
| 25. | Marrella splendens | 210 | |
| 26. | Marrella splendens | 212 | |
| 27. | Burgessia bella, Waptia fieldensis, and Opabinia regalis | 214 | |
| 28. | Opabinia regalis, Nathorstia transitans, and Naraoia compacta | 216 | |
| 29. | Molaria spinifera, Habelia optata, Yohoia tenuis, and Yohoia plena | 218 | |
| 30. | Bidentia difficilis, Emeraldella brocki, and Burgessia bella | 220 | |
| 31. | Hymenocaris perfecta and Leanchoilia superlata | 222 | |
| 32. | Hymenocaris obliqua, Hymenocaris ? circularis, Hymenocaris ovalis, Hymenocaris ? parva, Fieldia lanceolata, and Hurdia victoria | 224 | |
| 33. | Carnarvonia venosa and Tuzoia retifera | 226 | |
| 34. | Hurdia triangulata, Odaraia alata, and Anomalocaris gigantea | 228 | |
INTRODUCTION
This is the fourth preliminary paper based on collections from the Burgess shale member of the Stephen formation in British Columbia. The first paper described two genera of the Merostomata,[1] Sidneyia and Amiella; the second, the holothurians and medusæ, and the third the annelids.[2]
This paper includes all of the crustaceans of the subclasses Branchiopoda, Malacostraca, and Merostomata that occur in the collections of 1909 and 1910. A brief note is also given of some new features in the appendages of the Trilobita, and a few unusual forms of trilobites are noted by brief descriptions and simple illustrations. The few traces of the Ostracoda will not be noticed, and many details of structure of species are omitted, both in description and illustration, as I am planning to follow these preliminary notes with a paper on the Burgess shale fauna that shall include the results of a study of the present collections and those of the field seasons of 1911-1912.
Correction.—By oversight figures 2-4 of my paper on Middle Cambrian Holothurians and Medusæ, also the text references to Lankester's Treatise on Zoölogy, were credited to Lankester[3] instead of to F. A. Bather, the author of the section on the Echinodermata. Doctor Bather calls my attention to a paper by him in which he discusses the theoretical ancestor of the echinoderm.[4] Doctor Bather also informs me that Chapter XIII, which includes the Holothurioidea, was written by E. S. Goodrich, as stated in a footnote on page 218, Part III, of Lankester's Treatise on Zoölogy. This was also overlooked in citing from that portion of the treatise. Due credit will be given in the final paper on the subject of the Burgess shale fauna.
HABITAT
The crustaceans now found in the Burgess shale lived in quiet, relatively shallow waters swarming with life and readily accessible to the fauna of the open sea. In the preliminary study of the fauna I have distinguished 56 genera in collections from a block of shale not over 6 by 40 feet in area and 7 feet in thickness. Individuals of several species of crustaceans occur in large numbers at three horizons, notably Marrella splendens and Hymcnocaris perfecta. Trilobites, with the exception of the genera Agnostus and Microdiscus, are not abundant, although their tests almost make up calcareous shales a few feet below the base of the Burgess shale.
The compact, smooth, exceedingly fine-grained siliceous Burgess shale was deposited from relatively quiet, muddy water. At intervals this condition must have continued for some time as layers of the shale several inches in thickness have the crustaceans distributed irregularly through them. Where the shale is in thin layers with distinct lamination and bedding surfaces the fossils are more abundant but less perfectly preserved. The presence of carbonic acid gas at the surface of the mud has already been spoken of.[5]
Owing to faulting and alteration of the shales by shearing the area available for collecting is limited to about 120 feet of outcrop on a steep slope of the mountain. This condition limits our information as to the original extent of this remarkable mud deposit. It was probably laid down in a small bay or lagoon in close connection with the shallow Middle Cambrian sea.
CHARACTER OF THE SHALE
Mr. E. S. Larsen, Jr., of the United States Geological Survey, examined sections of the shale and from his notes the following is taken:
The microscopic examination of the thin section of the rock shows that it is very fine-textured—so fine that much of the material shows aggregate polarization. It is made up largely of white mica, which occurs in minute shreds or scales arranged parallel to the cleavage of the rock. Kaolinite is rather abundant and a very few minute grains of quartz, small prisms of apatite, and a few crystals of pyrite can be recognized. Numerous dark brown to black streaks arranged parallel to the cleavage represent carbonaceous matter. There is a system of parallel veinlets less than a millimeter across, which are normal to the slaty cleavage; fractures through the centers of these veins show small grains of calcite and blotches of cupriferous pyrite. The surfaces of a system of later fractures are irregular and are coated with carbonates. Sections of the veinlets mentioned are made up in large part of an isotropic mineral which is nearly colorless in the thin section. In the hand specimen it is pale green. It has an index of refraction of about 1.62 and preliminary chemical tests indicate that it is near the chlorites in composition. A further study of the mineral is being made. In the center of the veinlets are irregular crystals of calcite and a little pyrite.
A chemical analysis of the slate was made by Mr. George Steiger in the laboratory of the United States Geological Survey and is given under No. 1 of the following table. Analyses of several somewhat similar rocks and of a sericite are also given.
| 1 | 2 | 3 | 4 | 5 | ||
| SiO₂ | 54.49 | 55.80 | 60.28 | 57.96 | 55,00–67.00 | |
| Al₂O₃ | 25.60 | 27.72 | 22.61 | 24.70 | 11.00–23.00 | |
| Fe₂O₃ | 0.89 | 3.07 | 2.53 | 1.27 | 0.52–7.00 | |
| FeO | 2.00 | .... | 0.45 | 0.62 | 0.46–9.00 | |
| MgO | 1.18 | 0.53 | 1.35 | 2.16 | 0.88–4.57 | |
| CaO | 1.90 | 0.14 | 0.13 | 2.30 | 0.33–5.20 | |
| Na₂O | 0.28 | 1.51 | 0.54 | 6.95 | 0.50–3.97 | |
| K₂O | 6.67 | 5.62 | 5.73 | 2.56 | 1.76–5.27 | |
| H₂O– | 0.33 | .... | 0.60 | 0.04 | ||
| H₂O+ | 3.91 | 4.03 | 3.62 | 1.06 | 2.82–4.09 | |
| TiO₂ | 0.72 | .... | 0.69 | 0.88 | ||
| ZrO₂ | none | |||||
| CO₂ | 1.54 | |||||
| C | not det. | .... | 0.97 | |||
| P₂O₅ | 0.08 | .... | 0.03 | |||
| SO₃ | none | |||||
| S | 0.24 | |||||
| MnO | none | .... | tr. | |||
| BaO | none | .... | 0.04 | |||
| SrO | none | |||||
| CuO | tr. | |||||
| 99.83 | 98.42 | 99.57 | 100.50 | |||
| Less O | .09 | |||||
| 99.74 |
1. Middle Cambrian shale from British Columbia.
2. Sericite. Dürrberg. Quoted by Dana, System of Mineralogy, 6th edition, p. 618, analyses 41.
3. Mansfield slate (Lower Huronian). Crystal Falls District, Michigan. U. S. Geological Survey Monograph 36, p. 59.
4. Kata-biotite-orthoclase gneiss. Corundum-bearing. Waldheim, Saxony. Quoted from Grubenman, "Die Kristallinem Schiefer," 2nd edition, 1910, p. 158.
5. Range of composition of commercial slate of aqueous sedimentary origin according to Dale, U. S. Geological Survey Bulletin 275, p. 36.
The analysis shows a remarkable similarity to analysis 2, which is of the mineral sericite from Dürrberg; after deducting the calcite and pyrite from the slate analysis the similarity is still more striking. Analysis 3, which represents the Mansfield slate of Lower Huronian age from the Crystal Falls District, Michigan, is somewhat higher in silica and lower in aluminum but is otherwise very similar. Analysis 4 represents a kata-biotite-orthoclase gneiss, corundum-bearing, from Saxony, and differs from analysis 1 chiefly in its lower water content and in the relation between the soda and the potash. The fifth column gives the range of composition of commercial slates of aqueous sedimentary origin as given by Dale. The slate from British Columbia is outside of these limits in many respects; the silica is a little lower, the aluminum is high, the soda low, and the potash high. In general, this rock, as compared with other slates, phyllites, and related schists, is noteworthy for its low content in silica, its high aluminum and potash, and its poverty in all other oxides except water. The excess of potash over soda is especially remarkable.
The composition of the slate and its microscopic texture show that it was derived from a very fine, highly aluminous sediment, whose material must have consisted of the very finest suspended matter which had been leached unusually free from iron, magnesia, lime, etc., and which consisted largely of kaolinite and quartz.
It is interesting to note that Analysis 2 of the sericite is so similar to the Burgess shale, owing to the fact that where the Burgess shale is compressed and metamorphosed at the western end of the Burgess Pass beneath Mount Burgess it is to all appearances a sericiteschist. Owing to the Burgess shale member of the Stephen formation being overlain and underlain by massive limestones it is very frequently metamorphosed and cleaved into schists or soft calcareous or siliceous slates.
MODE OF OCCURRENCE
With the exception of Marrella splendens and Hymenocaris perfecta, Agnostus, and Microdiscus, the fossils are irregularly distributed and of relatively rare occurrence. They are pressed flat even in layers where there are no visible traces of lamination of the rock.
For convenience of reference I shall call the lower portion of the Burgess shale,[6] in which so many beautifully preserved fossils occur, the phyllopod bed, as it contains a large, unique, and fine series of phyllopod remains. It has a thickness of 7 feet, 7 inches, and is capped by a layer of coarse, bluish, dirty-gray shale weathering to a yellowish ochre-brown on the edges, that averages 18 inches in thickness. The phyllopod bed may be subdivided as follows from the top downward:
| ft. | in. | ||
| 1. | Bluish-gray siliceous shale with partings of dirty gray-colored shale | 1 | 9 |
| 2. | Dirty-gray shale | 0 | 8 |
| 3. | Bluish-gray shale in compact layers 3 to 4 inches thick | 1 | 0 |
| 4. | Dirty-gray shale | 0 | 2 |
| 5. | Bluish-gray, tough, brittle shale | 0 | 2 |
| Great Eldonia ludwigi layer. | |||
| 6. | Compact layer of bluish-gray hard rock that splits more or less evenly | 0 | 8 |
| 7. | Alternating dirty and bluish-gray shale | 0 | 9 |
| Great Hymenocaris perfecta bed. | |||
| 8. | The same character as No. 6: Compact layer of bluish-gray hard rock that splits more or less evenly | 0 | 8 |
| 9. | Dirty-gray, earthy shale | 0 | 2 |
| 10. | The same character as No. 6: Compact layer of bluish-gray hard rock that splits more or less evenly | 1 | 4 |
| This is one of the most important fossil-bearing layers—sponges, annelids, holothurians, and crustaceans. | |||
| 11. | Dark, dirty-gray, earthy shale | 0 | 1.5 |
| 12. | Bluish-gray, tough, brittle shale | 0 | 1.5 |
| This is the great Marrella splendens layer. | 7 | 7 |
Below No. 12 the layers of shale are arenaceous, irregular, and not favorable for preserving fine fossils.
In making the collections of 1910 and 1911 over 150 cubic yards of rock were quarried and split up. Frequently, however, many square feet of surface of the shale would be opened without exposing a desirable specimen.
Layer No. 12 is of great interest. It was a slab of this carried down by a snow slide that Mrs. Walcott and I found in 1909 on the trail from Burgess Pass to Summit Lake. It contains Marrella splendens in great numbers, and of the annelids it has yielded the only specimens of Miskoia preciosa and Amiskwia sagittiformis, and most of those of Pikaia gracilens, Wiwaxia corrugata, and Canadia spinosa. Among the crustaceans the only specimens of Opabinia regalis, Molaria spinifera, Yohoia tenuis, Y. plena, Mollisonia gracilis, and M. ? rara were found in it, and Burgessia bella, Waptia fieldensis, and Naraoia compacta are of more or less frequent occurrence.
Layer No. 10 gave many beautiful specimens, including several fine sponges and sertularians. Of the annelids, Ottoia prolifica, O. minor, Selkirkia major, S. gracilis, Oesia disjuncta, Pollingeria gracilis, Wiwaxia corrugata, Worthenella cambria, Asheaia pedunculata, Canadia spinosa, and C. setigera are present, and among the holothurians, Laggania cambria, Mackenzia costalis, and Louisella pedunculata. The medusa Pcytoia nathorsti also appeared at this horizon. The crustaceans include Marrella, Burgessia, Waptia, Nathorstia transitans, Naraoia compacta, Bidentia diMcilis, Emeraldella brocki, Leanchoilia superlata, Hymenocaris perfecta, H. obliqua, H. ? circularis, H. ovalis, H. ? parva, Tuzoia retifera Fieldia lanceolata, Hurdia victoria, H. triangulata, and Odaraia alata. Among the trilobites Neolenus serratus is found with its antennæ, caudal rami, branchiæ, and legs finely preserved.
No. 8 gave many plates of the annelid Pollingeria grandis and several specimens of the large Odaraia alata. In the dirty-gray layer of No. 9 the large Anomalocaris gigantea occurred.
In layer No. 5 the pelagic holothurian Eldonia ludwigi was abundant over a limited area, and also Marrella splendens and Hymenocaris perfecta.
Above No. 5 the scattered valves of Hymenocaris perfecta and more or less imperfect annelids (Ottoia prolifica, Pollingeria grandis, and Banffia grandis) were occasionally found, along with sponges, brachiopods, and fragments of trilobites. The small gastropod Scenella varians is found throughout the phyllopod bed and often its depressed conical shell, with the apex up, occurs in great numbers.
The mode of occurrence and limited area of the fauna indicate that we have only a portion of a crustacean fauna that was already developed early in Cambrian time and whose descendants swarmed in the Silurian and Devonian seas.
CLASSIFICATION
The classification used is partly that of Dr. W. T. Calman as outlined in Lankester's Treatise on Zoölogy, Part VII, 1909, and such additions as I have found it necessary to make in describing the many unique forms from the Burgess shale. All of the genera described in this paper fall under the subclasses Branchiopoda, Malacostraca, Trilobita, and Merostomata, and existing orders.
Table of Classification
Class Crustacea
Sub-Class Branchiopoda
Order Anostraca Calman
Family Opabinidæ, new family
Genus Opabinia, new genus
Genus Leanchoilia, new genus
Genus Yohoia, new genus
Genus Bidentia, new genus
Order Notostraca Calman
Family Naraoidæ, new family
Genus Naraoia, new genus
Family Burgessidæ, new family
Genus Burgessia, new genus
Family (Undetermined)
Genus Anomalocaris Whiteaves
Family Waptidæ, new family
Genus Waptia, new genus
Sub-Class Malacostraca
Order Hymenocarina Clarke
Family Hymenocaridae Salter
Genus Hymenocaris Salter
Family (Undetermined)
Genus Hurdia, new genus[7]
Genus Tuzoia, new genus
Genus Odaraia, new genus
Genus Fieldia, new genus
Genus Carnarvonia, new genus
Sub-Class Trilobita
Order (Undetermined)
Family Marrellidæ, new family
Genus Marrella, new genus
Family (Undetermined)
Genus Nathorstia, new genus
Order Hypoparia Beecher
Family (Undetermined)
Genus Mollisonia, new genus
Genus Tontoia, new genus
Sub-Class Merostomata
Order Aglaspina, new order
Family Aglaspidæ Clarke
Genus Molaria, new genus
Genus Habelia, new genus
Genus Emeraldella, new genus
Order Limulava Walcott
Family Sidneyidæ Walcott
Genus Sidneyia Walcott
STRATIGRAPHIC DISTRIBUTION
The several genera of the four subclasses (with the exception of the group of malacostracans represented by genera other than Hymenocaris and the trilobitic genera Mollisonia and Tontoia) have approximately the known vertical range in the Cambrian noted in the diagram on page 156.
In addition to representatives of the subclasses Branchiopoda, Malacostraca, Trilobita, and Merostomata, mentioned in this paper, I have added in the table genera of the Merostomata that occur in the Lower Cambrian and Algonkian, respectively, and of the Ostracoda in the Lower Cambrian, in order to present an outline of the lowest known stratigraphic position of the five subclasses of Crustacea. With the exception of the Branchiopoda all of these are known to have representatives in later Paleozoic formations.
The subclass Merostomata is represented by Beltina[8] in the pre-Cambrian; by Amiella[9] in the upper part of the Lower Cambrian, by the latter genus and Habelia, Molaria, Emeraldella, and Sidneyia[10] in the Middle Cambrian Burgess shale; and by Aglaspis[11] and Strabops[12] in the Upper Cambrian.
The Phyllocarida is first known in the Lower Cambrian by Isoxys,[13] a genus that is represented in the Burgess shale. Hymenocaris is well distributed in the lower half of the Middle Cambrian and the order Hymenocarina continues on up into the Ordovician, Silurian, and Devonian.

Stratigraphic Distribution of Earliest Representatives of Each of the Five Subclasses of the Crustacea.
The Trilobita begins with Nevadia deep down in the Lower Cambrian[15] and predominates in all later Cambrian faunas.
I do not know of any genera of the Branchiopoda in the Cambrian other than those described in this paper from the Burgess shale and the single specimen of Protocaris from the upper part of the Lower Cambrian.[16]
That a large and varied crustacean fauna preceded and followed that of the Burgess shale is certain, and large additions to our information of it will undoubtedly be forthcoming in the near future.
STRUCTURAL FEATURES
Exoskeleton.—Among the Anostraca there is no true shell, the external cuticle being little more than a membrane that is thicker in the cephalic region and on the telson, if the latter is present. Among the notostracans the carapace varies from the simple form seen in Burgessia (pl. 27) to the double shield of Naraoia (pl. 28). The malacostracans all have a strong bivalve carapace, as shown on plates 31-34.
The carapace of Marrella (pl. 25, fig. 1) is most interesting. The eyes on the anterior margin, the large antennules (?), and the great posterior dorsal spines indicate a great modification of and advance over the simple primitive shield resulting from a fold of the cuticle of the fifth segment of the head. The shield of Burgessia (pl. 27, figs. 1-3) is simple, and that of Naraoia (pl. 28, fig. 4) simple over the head and more complex over the thorax.
In Waptia (pl. 27, figs. 4 and 5) the shield has passed nearly to the bivalve stage of the Hymenocarina. It appears to be a transition between the simple bent shield of Burgessia and the bivalve carapace of Hymenocaris (pl. 31).
The bivalve carapaces of Tusoia and Carnarvonia are so similar to the carapace of living forms of the Nebaliacea that there is little question of the intimate relationship between them. The reticulated surface on the large carapaces of Carnarvonia (pl. 33, fig. 1) and Tuzoia (pl. 33, fig. 2), also approximates in pattern and size that of Nebaliopsis typica Sars.[17]
The merostomes Sidneyia,[18] Amiella,[19] Habelia (pl. 29), Molaria (pl. 29), and Emeraldella (pl. 30), have a compact cephalic shield and well-defined thoracic and abdominal segments and telson that are similar in character to the dorsal shield of the trilobite.
Labrum.—The labrum or hypostoma of Sidneyia[18] Emeraldella (text fig. 8, p. 204), and Marrella (pl, 26, fig. 2) is clearly shown in a number of specimens, also that of Burgessia (pl. 27, fig. 2), but in the other species it has not been seen nor has the lower lip (labium or metastoma) been observed in any species.
Segmentation.—The following table gives the number of cephalic, thoracic, and abdominal segments so far as known. The eyes are considered as representing a segment, which gives six segments in the cephalic region.
Table of Cephalic, Thoracic, and Abdominal Segments.
| Genera. | Cephalic segments. |
Thoracic segments. |
Abdominal segments. |
| BRANCHIOPODA. | |||
| Opabinia | ? | 16 | 1 |
| Leanchoilia | 4¹ | 9 | ? |
| Yohoia | 6 | 8 | 4 |
| Bidentia | ? | 11 | 1 |
| Naraoia | 6² | 17-19 | 3 |
| Burgessia | 6 | 8 | 30+ |
| Anomalocaris | .. | ? | 12+ |
| Waptia | 6 | 8 | 6 |
| MALACOSTRACA. | |||
| Hymenocaris | 6 | 8 | 6? |
| TRILOBITA. | |||
| Marrella | 6 | 24 | 1 |
| MEROSTOMATA. | |||
| Sidneyia | 5 | 9 | 3 |
| Habelia | 6?² | 11 | 2 |
| Emeraldella | 3 | 11 | 3 |
| Molaria | 6² | 8 | 2 |
¹ All that have been seen on imperfect specimens.
² The eyes are considered in this table as representing a cephalic segment.
In the table the telson has been included as an abdominal segment and the caudal rami are considered as attachments of the terminal segment.
Appendages.—So far as can be determined from the specimens now available for study the normal number of cephalic appendages of the Branchiopoda and Malacostraca is six if we consider the stalked eyes as representing the first pair.
| Eyes = first Antennules = second Antennæ = third Mandibles = fourth Maxillulæ = fifth Maxillæ = sixth |
The stalked eyes are distinctly shown for Opabinia (pl. 28, fig. 1), Waptia (pl. 27, fig. 4), and Yohoia (pl. 29, fig. 9), and for Hymenocaris by specimens not illustrated. Burgessia (pl. 27) and Marrella (pl. 25, figs. 4 and 5) have sessile eyes and five pairs of cephalic appendages. The sessile eyes, as in the trilobite, probably represent a segment of the cephalic shield.
The character of the several thoracic appendages is described under each species. So far as determined, the stalked eyes, antennules, and antennæ are not very unlike those of recent crustaceans of the same orders, and the mandible, maxillula, and maxilla also have the same fundamental structure with modifications to meet the needs of each genus and species.
The thoracic appendages appear to be based on the typical crustacean limb having a protopodite bearing an exopodite and endopodite. There are no recognized modifications of this that would indicate a simpler form. An epipodote (gill) is attached to the protopodite in Marrella (pl. 26, fig. 4), Opabinia (pl. 27, fig. 6), and Malaria (pl. 29, fig. 3).
The number of thoracic appendages is indicated in the table (p. 158) which gives the number of thoracic segments. Each of these thoracic segments is considered to have had a pair of attached appendages although, as in the case of Apus, the posterior segments may possibly have had more than one pair of appendages.
Simple, bifid abdominal appendages only appear on the abdominal segments of Anomalocaris (pl. 34, fig. 3). The caudal rami of the abdominal segment vary greatly in form and structure in the crustaceans from the Burgess shale. The female of Opabinia appears to have two unsegmented, expanded rami. Waptia (pl. 27, figs. 4 and 5) and Yohoia (pl. 29, figs. 8, 11, and 14) have two expanded rami with rudimentary segmentation. Hymenocaris (pl. 31, figs. 3 and 5) and Odaraia (pl. 34, fig. 2) have several cercopods attached to the last abdominal segment, and the trilobite Neolenus (pl. 24, figs. 1 and 1a) has two long, slender, jointed rami.
Most of the Branchiopoda are provided with strong, broad, setiferous swimming exopodites that probably also served in Marrella (pls. 25 and 26), Opabinia (pl. 27, fig. 6, and pl. 28, fig. 1), and Leanchoilia (pl. 31, fig. 6) to bring food to the mouth.
In a future paper I expect to illustrate and describe in detail the appendages of each species so far as the material will permit.
Alimentary canal.—This has been preserved in a number of species. In Opabinia (pl. 28, fig. 1), Marrella (pl. 26, fig. 6), and Burgessia (pl. 27, figs. 1 and 2), it is straight from the head to its posterior end and expands more or less in the cephalic region. The size of the canal varies from the head to the anus as in Opabinia (pl. 28, fig. 1) and Marrella (pl. 25, fig. 6 and pl. 26, fig. 6) but how much this may be due to flattening in the shale is uncertain. In Burgessia (pl. 27, figs. 1 and 2) the canal is large at the point where the hepatic tubes join it, and tapers to its posterior end. It is rounded as though retaining its contents in a fossil state. This is also true of the slender rounded canal of Hymenocaris (pl. 31, fig. 2). In Burgessia the hepatic tubes enter it back of the maxillæ. The stomach is indicated by the expansion of the anterior end of the alimentary canal. It is also outlined by a slight contraction of the canal (pl. 27, fig. 1).
Hepatic cæca.—The hepatic cæca are beautifully preserved in the shield of Burgessia (pl. 27, figs. 1-3), Naraoia (pl. 28, fig. 4), and Molaria (pl. 29, fig. 3). In Burgessia they reach their greatest development, the branches showing in fine detail on the dark shale. No definite structure has been detected in the dorsal spines of Marrella (pl. 26, fig. 1) that could certainly be referred to as the glands, but the fact that the spines have a relatively large central canal suggests that they may have contained them.
Among recent crustaceans the hepatic cæca are branched in some copepods, Corycæidæ and Asterocheridæ, but none have the beautiful structure found in Burgessia. We called the latter the "Kidney crab" in camp on account of the shape of the cæca, but as the cæca open directly into the alimentary canal they could hardly function as kidneys.
ORIGIN OF MIDDLE CAMBRIAN CRUSTACEAN FAUNA
The Cambrian crustacean fauna suggests that five (p. 156) main lines or stems (Branchiopoda, Malacostraca, Ostracoda, Trilobita, and Merostomata) were in existence at the beginning of Cambrian time and that all of them had already had their inception in Lipalian time or the period of pre-Cambrian marine sedimentation of which no known part is present on the existing continents.[20]
The known stratigraphic position of the various genera is shown by the diagram on page 156. In this 13 genera are found only at one limited horizon (phyllopod bed) in the Middle Cambrian. The five subclasses are represented as having had a long line of crustacean ancestors, a view that if correct would manifestly necessitate a prolonged pre-Cambrian period for the development of the crustacean fauna now found in the Burgess shale. As the trilobites are

Theoretical Lines of Descent of Cambrian Crustacea
probably derived from the same stock as the Branchiopoda, the lines of probable descent of the various genera of the latter in the Burgess shale are projected backward into the pre-Cambrian. It may be that some of the genera of the Branchiopoda in the table were developed in early Cambrian time, but of this we have no evidence.
A suggested scheme of descent of the genera in the table and other Cambrian genera, with the exception of the genera of the Trilobita, is shown in the above diagram.
The Lower Cambrian formations have only been searched in a very superficial manner in those parts of North America where they are well developed and finely exposed for the collection of fossils. This leads me to think that it is only a question of time and detailed work to bring to light a large and varied crustacean fauna. This almost certainly existed, as proven by the occurrence of Beltina in the pre-Cambrian of the Rocky Mountains.[21]
Bernard's very interesting and valuable study of Apus[22] is replete with suggestions and inferences bearing on the evolution of the Crustacea from a browsing carnivorous annelid with its first 5 segments (head) bent so that its mouth faced ventrally and posteriorly, and using its parapodia for pushing food into its mouth. He concludes that the modern representative of this crustacean-annelid is Apus. With Bernard's theory in mind I have examined the Burgess shale annelidan and crustacean fauna to ascertain if there was an annelid that could be considered as representing his hypothetical crustacean-annelid, and nearer to it in structure than Apus. I found specimens of Canadia spinosa Walcott laterally flattened in the shale with the head bent down, so that the mouth faces posteriorly,[23] also that 14 out of 24 specimens have the head bent under and out of sight beneath the flattened body. Possibly these annelids and the crustaceans were derived from the same general type of animal.
Among the crustaceans Marrella splendens (pls. 25 and 26) has an Apus-like form, but it is evidently a more highly developed form than Apus. This is shown among other characters by its carapace, long jointed legs, and fewer segments.[24] Burgessia bella (pl. 27, figs. 1-3) has a simple carapace, few thoracic segments, and many abdominal segments, if those of the telson-like extension of the body are considered as belonging to the abdominal region. The eight thoracic segments serve to separate Burgessia from Apus and other genera of the Apodidæ and at the same time bring it near to the Phyllocarida as represented by Nebalia. On the other hand, the simple Lepidurus-like carapace, sessile eyes, and hepatic glands in the carapace serve to place Burgessia in the Branchiopoda under the order Notostraca.
Among the anostracans Opabinia regalis, in its elongate many-segmented body, phyllopod-like swimming exopodites and insignificant or rudimentary ambulatory endopodites, small head, and slender body, is very suggestive of an annelidan ancestor.
These comparisons raise the question as to the relations of the Branchiopoda, Leptostraca (representing the Malacostraca), Trilobita, and Merostomata. With the data afforded by the Burgess shale fauna the inter-relationship of the four so-called subclasses is found to be very intimate.
In Opabinia (pl. 27, fig. 6, and pl. 28, fig. 1) and Leanchoilia (pl. 31, fig. 6) the typical branchiopod is clearly present.
In Waptia (pl. 27, figs. 4 and 5) the Leptostraca is very near at hand as developed in Hymenocaris (pl. 31, figs. 1 and 2).
In Marrella (pls. 25 and 26) the trilobite is foreshadowed, and Nathorstia (pl. 28, fig. 2) is a generalized trilobite as the trilobite appears to be a specialized branchiopod, adapted largely for creeping on the bottom. The trilobite gives some conception of a possible form between the Branchiopoda and the Aglaspidæ of the Merostomata.
Such forms as Habelia (pl. 29, fig. 6), Molaria (pl. 29, figs. 1-5), and Emeraldella (pl. 30, fig. 2) serve to fill in the gap between the Branchiopoda and the Merostomata as represented by Sidneyia[25] and later the Eurypterida. Sidneyia is now known to have a pair of jointed biramous appendages on each of the anterior 9 segments of the body. The inner division or endopodite is a jointed leg adapted for creeping close to the bottom and the outer branch is a lamellated branchial lobe (see Smithsonian Misc. Coll., vol. 57, No. 2, 1911, pl. 6, fig. 3, and pl. 7, fig. 1; and text fig. 10 of this paper, p. 206).
In the following diagram the attempt is made to show the relations of Cambrian crustaceans to a theoretical ancestral stock which for convenience is correlated with the Apodidæ. From this stock it is assumed that the Branchiopoda came, and from the Branchiopoda stock three distinct branches were developed prior to or during Cambrian time. Of these the one of greatest interest in the present connection is that on the right of the diagram. In this line of descent it is assumed that the Trilobita are directly descendent from the Branchiopoda and forms grouped under the order Aglaspina derived from the Trilobita. The order Limulava is considered as being intermediate between Aglaspina and the Eurypterida, and that the two orders Limulava and Aglaspina serve to connect the Trilobita and the Eurypterida.
From the Eurypterida we pass to the Xiphosura. It is thought that the Phyllocarida, as represented by the group of forms included under the Hymenocarina, came from the Branchiopoda, but on a different line of descent from the Trilobita and the orders grouped under the Merostomata.
The ostracods are assumed to have been derived from the Branchiopoda but on a different line of descent from the Trilobita and Phyllocarida.

Theoretical Evolution of Cambrian Crustacea from the Branchiopoda
I will not attempt further to discuss the various lines of descent of the genera in this preliminary notice, as in the spring of 1913 much more material may be available for study. The outline diagram (p. 161) indicates my present view, though this is tentative pending study and comparison with living forms. Any speculation on the origin of the various invertebrate groups based on the faunas found in the Cambrian must necessarily be very defective, as the pre-Cambrian development extended far back into pre-Cambrian time.
RELATION TO RECENT CRUSTACEANS
That the Burgess shale crustacean fauna was a tremendous surprise to me and that it will be to all paleozoologists is evident to any one acquainted with what was known of the early Paleozoic Crustacea and the theoretical views concerning its development. The highly organized merostome Sidneyia inexpectans[26] removed the origin of the Merostomata far back into pre-Cambrian time and seemed to link the problematic Beltina of the Algonkian Belt terrane with the merostomes of Ordovician and Silurian time, and through them with the living Ziphosuridæ. That Branchiopoda of the order Anostraca lived in Cambrian time is not so surprising, but that they should be almost perfectly preserved, and closely allied to the living forms, certainly is unexpected. Opabinia regalis (pl. 27, fig. 6, and pl. 28, fig. 1) is much like Thamnocephalus platyurus Packard,[27] and Burgessia (pl. 27, figs. 1-3) has the dorsal shield and somewhat similar cephalic region of Lepidurus.
Hymenocaris (pl. 31) may be compared with Nebalia, and Carnarvonia (pl. 33) and Tuzoia (pl. 33) with the reticulated carapace of Nebaliopsis typica Sars.[28]
The group of forms represented by Nathorstia (pl. 28, fig. 2), Naraoia (pl. 28, fig. 4), Yohoia (pl. 29, figs. 7-14), and Bidentia (pl. 30, fig. 1) does not appear to have any living representatives.
Viewed as an ancestral fauna of the living Crustacea the Burgess shale fauna foreshadows the Branchiopoda in both its orders, Anostraca and Notostraca; the Ostracoda by the family Indianidæ[29]; the Malacostraca by the Phyllocarida; and the Merostomata by Aglaspina and Limulava.[30]
SURVIVAL OF THE BRANCHIOPODA
The recent Polyartemidæ and Apodidæ are animals that by their remarkable adaptation to conditions are practically immune to agencies that, during geologic time, have destroyed whole races of invertebrate animals. When they became adapted to living in intermittent ponds that depended on rainfall and that might be fresh, brackish, or saline, is unknown. Their wide geographic distribution and the great vitality of their eggs indicate great age, and the discovery of their probable ancestors in such forms as Opabinia (pl. 27, fig. 6, and pl. 28, fig. 1) and Burgessia (pl. 27), in association with a large and varied Middle Cambrian fauna, proves that in that early time they were capable of flourishing in the midst of active and powerful enemies. This was owing undoubtedly to their great power of reproduction and active movements.
Bernard[31] attributes the preservation of the Apodidæ in geologic time to the isolated manner of life of the animals. This may be true since Carboniferous time, but I doubt if it was so during the long, early Paleozoic ages. The evidence for the existence of a land surface since early Carboniferous time with continuing streams or ponds is found in the presence in Lower Carboniferous strata of fresh-water shells that were undoubtedly the ancestors of the living fresh-water genera Physa[32] and Ampullaria.[33] It may be that the descendants of the Cambrian Branchiopoda became adapted to fresh-water conditions in Devonian time after the disappearance of the large group of merostomes that reached its greatest development and almost disappeared in Silurian time.
That the smaller and more delicate forms of the Branchiopoda have not been found in Ordovician, Silurian, and later rocks is no proof that they did not exist side by side with the thick shell-covered crustaceans that have only left traces here and there in the sediments.
Class CRUSTACEA
Sub-Class BRANCHIOPODA
Order ANOSTRACA Calman[34]
OPABINIDÆ, new family
Carapace absent; paired eyes pedunculate; antennæ unknown, frontal appendage (proboscis) flexible, prehensile in male, bifid in female. Trunk limbs 16 pairs, the terminal joints of the feet broad and spatulate as in the Thamnocephalinæ, Abdomen a simple plate, with two caudal, unsegmented furcal rami on the female.
The Opabinidæ differ from the most nearly allied family, Thamnocephalinæ Packard, in having a simple plate-like unsegmented abdomen.
OPABINIA, new genus
The generic and specific descriptions are united under the description of the species.
Genotype.—Opabinia regalis, new species.
Stratigraphic range.—The stratigraphic range is limited to a band of dark siliceous shale about 4 feet in thickness, forming a part of the Burgess shale member of the Stephen formation.
Geographic distribution.—On the slope of the ridge between Wapta Peak and Mount Field, north of Burgess Pass, and about 3800 feet above Field on the line of the Canadian Pacific Railway, British Columbia, Canada.
The generic name is derived from Opabin, the name of a pass between Mount Hungabee and Mount Biddle, southeast of Lake O'Hara, British Columbia, Canada.
OPABINIA REGALIS, new species
Plate 27, fig. 6, and plate 28, fig. 1
Body elongate, moderately wide, and divided into a small head section, a trunk of 16 somites, and a broad telson. The base of the head is formed of an elongated portion about as wide as long when flattened in the shale; in front of this the head narrows where the base of a large stalked eye is attached on each side. In front there is a short section from which a strong central appendage extends directly forward as viewed from above (fig. 1, pl. 28) and curves upward from the front lower side of the head when seen in profile (fig. 6, pl. 27). The appendage is narrow, wrinkled, and more or less flexible; near the anterior end it expands to form a base of attachment of a number of small, slightly incurved, short claws or spines.
The eyes are at the end of a strong, short stalk and traces of the reticulated surface of the compound eye remain on the matrix of the specimen illustrated by figure 1, plate 28.
The 16 somites of the post-cephalic body (thorax) are very uniform in appearance and size except that the posterior somites gradually decrease in size and width. Their arrangement is finely shown in profile view by figure 6, plate 27.
The terminal somite is a broad, elongate, spatulate lobe with a short point on each postero-lateral rounded angle. Between the points there is a transverse line that may mark a division of the telson and the presence of a post-anal plate.
Appendages.—The anterior, central cephalic appendage has been mentioned. It suggests the appendage of the male of the species. Reference to the possible presence of the female in the collection will be spoken of later.
None of the heads of the four specimens show traces of antennules, antennæ, mandibles, or maxillæ. If these appendages were large they have been broken off; if small they may be concealed beneath the crushed and flattened large posterior section of the head.
The thoracic legs are shown both in side view (fig. 6, pl. 27) and from below on a flattened specimen (fig. 1, pl. 28). They appear to be of a uniform character on all the 16 somites except the two anterior pairs, which may be smaller and have narrower terminal joints. The legs are formed of two or three rather strong, short joints followed by broad, flat, elongate-oval lobe-like joints (f, fig. 6, pl. 27). The gills are shown as oval lobes on the upper portion of the leg (br, fig. 6, pl. 27). The terminal elongate swimming joint or fin is shaped much like that of the common Branchipus vernalis Verrill. A strongly setiferous lobe occurs above the large terminal joint, but its relations to it are not clear. Another feature difficult to interpret is that of the groups of short, longitudinal lines shown in figure 1, plate 28. My present view is that they are groups of strong setæ attached to one or more of the lobe-like middle joints of the leg. I obtained an almost similar effect by pressing flat between glass plates a specimen of the recent Branchinecta paludosa (O. F. Miiller).
The details of structure of the leg cannot be determined, but judging from the material available for examination they follow somewhat closely the leg of Thamnocephalus as illustrated by Packard.[35]
Interior structure.—The alimentary canal is readily traced from the head back to the posterior portion of the terminal lobe between the two points (fig. 1, pl. 28). Parts of the canal are convex and presumably contain portions of the matter in the canal at the time of the death of the animal.
A very beautiful specimen showing some details of the interior has recently been worked out, but with the chance of getting more satisfactory specimens before a more complete review of the Burgess shale fauna is prepared I will not attempt to interpret its somewhat confused structure.
Dimensions.—The four specimens in the collection have the following longitudinal dimensions in millimeters:
| Length. | Proboscis. | Head. | Trunk. | Telson. | |
| No. 1 | 86 | 24 | 9 | 44 | 9 |
| No. 2 | 78 | 16 | 10 | 45 | 7 |
| No. 3 | 72 | 20 | 8 | 37 | 7 |
| No. 4 | .. | 24 | 9 | 51 | Broken |
Female.—There are two associated specimens that I have referred to the female of this species. One has a length of 61 mm. and the other of 52 mm., exclusive of any frontal appendages. The female differs from the male in having two slender caudal appendages or rami; and in having a slender bifid frontal appendage instead of the strong appendage of the male. The character of the frontal appendage is more or less doubtful as it is turned under and back on the side of the body. I hope that we will find in the collections of the summer of 1912 specimens that will add much to our knowledge of all parts of both the male and female of this species.
Observations.—Compared with recent forms Opabinia regalis has many outward characters of Thamnocephalus platyurus Packard.[36] The proboscis, form of head, body segments, and expanded terminal segment or telson are very suggestive of Thamnocephalus. So far as can be determined the structure of the thoracic legs is essentially similar, but this of course is subject to revision. After flattening specimens of Branchinecta and Branchipus between plates of glass and studying them, I am greatly surprised that any distinct characters of the appendages are preserved in the fossils in a recognizable condition.
The frontal appendage is referred to as the proboscis. It is united directly with the front of the head; it was flexible and provided with a central canal that may be traced from its base out to the expanded end, which has a circle of small, curved claw-like spines attached to it. The function of the proboscis and its terminal spines is unknown; it appears to be adapted to the gathering of food and conveying it to a mouth beneath the head, but it was probably used by the male to seize the female.
If we consider the appendage-bearing somites as the thorax, the abdomen is confined to the one elongated expanded somite I have referred to as the telson. This does not show evidence of segmentation unless there is a post-anal plate, which is very doubtful. None of the specimens of the male show any traces of caudal appendages.
Formation and locality.—Middle Cambrian: (35k) Burgess shale member of the Stephen formation, on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.
OPABINIA ? MEDIA, new species
This species was not recognized until after the plates illustrating the crustaceans from the Burgess shale had been completed.
It differs from Opabinia regalis (pl. 27, fig. 6) (a) in being much smaller, (b) in having a proportionately smaller head, and (c) in having fewer segments, 12 or 14. The frontal appendage is not clearly shown, but it is small compared with that of O. regalis.
The appendages of the thorax have an expanded setiferous terminal joint, and there are traces of a small, broad, lanceolate gill or flabellum toward the basal part of the leg.
The two largest specimens each have a length of about 38 mm. The specimens of this species are not well preserved, but the characters are sufficiently clear to distinguish the species from O. regalis. A thorough search will be made for better specimens during the season of 1912.
Formation and locality.—Middle Cambrian: Burgess shale member of the Stephen formation (about 75 feet above the phyllopod bed near the base of the shale) on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.
LEANCHOILIA, new genus
The generic description is included with that of the type species.
Genotype.—Leanchoilia superlata, new species.
Stratigraphic range.—The stratigraphic range is limited to a band of dark siliceous shale about 4 feet in thickness forming a part of the Burgess shale member of the Stephen formation.
Geographic distribution.—On the slope of the ridge between Wapta Peak and Mount Field, north of Burgess Pass, and about 3800 feet above Field, on the line of the Canadian Pacific Railway, British Columbia, Canada.
The generic name is derived from Leanchoil, the name of a railway station on the Canadian Pacific Railway, 17 miles southwest of Field, British Columbia, Canada.
LEANCHOILIA SUPERLATA, new species
Plate 31, fig. 6 (lower specimen)
Body elongate, with clearly defined head shield and nine strong body segments up to the point where the posterior part of the body is broken off. The anterior pointed end of the head is broken off in such a manner that the presence of a frontal appendage is suggested. The large opening on the side of the head indicates a large pedunculated eye comparable with that of Opabinia regalis (pl. 28, fig. 1).
Appendages.—Of the head appendages, the antennæ are the best preserved. These are large and composed of several strong joints of which three now show from beneath the carapace; the second of these bears a long slender branch on its inner margin, and the third two branches, one of which is similar to that of the second joint. These two branches appear to be composed of one very long slender joint followed at the end by several very short small joints that curve upward and presumably gave the branches flexible extremities; the third and lower branch has a similar slender proximal joint that at its outer end has three slender, jointed branches. This structure makes a very effective clasper of each of the antennæ. Back of the right antenna are two narrow appendages that may be the ends of one of the third and fourth pairs of head appendages.
The thoracic legs terminate in flat, elongate, broad, lanceolate joints. The terminal joint is about three-fifths the entire length of the leg, and has a fringe of strong setæ on its outer and posterior margin. The condition of preservation is such that the details of structure of the other portions of the leg cannot clearly ba determined.
The size and proportions of the type and only example of the species are shown by the lower specimen of figure 6, plate 31.
Observations.—This is one of the rare species in the collection. The anterior half was found after a dynamite blast and later the matrix showing the posterior portion and part of the anterior was picked out of the débris. Working as we often did with cold rain or snow falling, fragments once lost trace of were rarely recovered.
The large natatory, distal joints of the thoracic legs are much like those of Opabinia regalis (fig. 6, pl. 27), also the large eye. For the present the species is placed in the family Opabinidæ, although I fully realize that the reference is of the most tentative character.
Formation and locality.—Middle Cambrian: (35k) Burgess shale member of the Stephen formation, on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.
YOHOIA, new genus
The description of Yohoia tenuis embodies the characters of the genus.
Genotype.—Yohoia tenuis, new species.
Stratigraphic range.—The stratigraphic range is limited to a band of dark siliceous shale about 4 feet in thickness forming a part of the Burgess shale member of the Stephen formation.
Geographic distribution.—On the slope of the ridge between Wapta Peak and Mount Field, north of Burgess Pass, and about 3800 feet above Field on the line of the Canadian Pacific Railway, British Columbia, Canada.
The generic name is derived from Yoho, the name of the beautiful Yoho Valley, east of Mounts Wapta and Field.
Observations.—Two species have been referred to the genus, Y. tenuis and Y. plena. Both are elongate, slender, and have a small cephalic carapace, eight thoracic and four abdominal segments, with expanded caudal rami on the posterior segment.
YOHOIA TENUIS, new species
Plate 29, figs. 7-13
Body elongate, slender. Head short and without a carapace. Thorax with eight segments. Abdomen with four segments, the posterior bearing a pair of expanded caudal rami.
Head sub-quadrangular in outline, composed of five coalesced segments, the posterior four of nearly equal width and the anterior narrow. The segmentation of the head is very plainly shown on some specimens (fig. 12) and not on others (fig. 10). I have inclined at times to consider that there was a cephalic carapace, but finally decided that if present it was very small and thin and not to be recognized as a true carapace. The eyes are small, pedunculated, and rarely seen, since they appear, in side view, to be in a niche between the first and second segments of the head. As seen from above, on a specimen from which the edge of the test has been removed, they are small, round, bright spots (fig. 9).
The thorax is composed of eight segments that, with triangular-shaped pleurons on each side, clearly separate the thoracic segments from the four cylindrical segments of the abdomen. The two expanded rami attached to the posterior abdominal segments were thin and readily distorted by compression in the shale.
Appendages.—The first pair (antennules) appear to be short and blunt as they project beyond the anterior end of the head (fig. 13, side view; fig. 9, top view). The second pair (antennæ) have several joints (three are shown beyond the margin of the head) with a terminal group of three long, slender, curved spines (fig. 13). These probably represent the claspers of the male. The third, fourth, and fifth cephalic appendages show as small jointed legs hanging below the head.
The appendages of the thorax are not very well preserved. They indicate a leg much like that of Waptia fieldensis (pl. 27, fig. 5), composed of broad joints, the last provided with numerous long setæ.
No appendages or setæ have been observed on the four abdominal segments.
Very little is known of the interior structure, except the presence of a slender, straight alimentary canal. One specimen, as viewed from above (fig. 9), suggests a division into two lobes of the interior of the head.
Dimensions.—The largest specimen has a length of 24 mm. The other dimensions as the animal is flattened in the shale are shown by the figures on plate 29.
Observations.—This species is associated with Waptia fieldensis (pl. 27, figs. 4 and 5) and has the same type of body and expanded caudal rami. It differs in the absence of a carapace; in having four instead of six abdominal segments; and so far as known a different form of antennæ.
Formation and locality.—Middle Cambrian: (35k) Burgess shale member of the Stephen formation, on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.
YOHOIA PLENA, new species
Plate 29, fig. 14
This species has a proportionately larger head, thicker body with shorter segments, and the caudal rami are more expanded than in the associated Yohoia tenuis. A somewhat similar form from about 75 feet higher in the Burgess Shale is represented by two imperfect specimens.
Specimens of this species reach a length of 24 mm., but most of them are about half as long.
Formation and locality.—Middle Cambrian: (35k) Burgess shale member of the Stephen formation, on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.
BIDENTIA, new genus
The description of the genus is included with that of the type species.
Genotype.—Bidentia difficilis, new species.
Stratigraphic range.—The stratigraphic range is limited to a band of gray siliceous buff-weathering shale forming a part of the upper portion of the Burgess shale member of the Stephen formation.
Geographic distribution.—On the slope of the ridge between Wapta Peak and Mount Field, north of Burgess Pass, and about 3800 feet above Field on the line of the Canadian Pacific Railway, British Columbia, Canada.
The generic name is derived from Bident, the name of one of the mountain peaks east of the "Valley of the Ten Peaks," south of Laggan, Alberta, Canada.
BIDENTIA DIFFICILIS, new species
Plate 30, fig. 1
Body elongate, with well-marked head, thoracic segments, and expanded caudal rami. Head short and, as pressed flat on the shale, semicircular with the straight side jointed to the thorax. In figure 1 I have dotted the approximate outline of the head. Thoracic segments short and of nearly equal length; there appear to be eleven that have attached appendages. Abdomen with one segment and a pair of expanded rami. The latter are pressed together in figure 1; in another specimen, not illustrated, they are more flattened out.
Appendages.—The only appendages of the head shown by figure 1 are the strong antennæ (a'). They have a thick, jointed basal portion with two long jointed branches. The latter may be the claspers of the male.
The thoracic limbs are obscure owing to the great pressure and flattening they have undergone. Those best preserved along the central segments show a large, broad lanceolate terminal segment fringed with long setæ on the posterior margin; gill lobes are indicated on the upper portion of the leg.
Dimensions.—The largest specimen has a length of 45 mm. exclusive of the telson which is about 10 mm. long.
Observations.—At first I placed this species with Emeraldella brocki (pl. 30, fig. 2), but further study of the specimen illustrated and one other led to its separation as the type of a new genus and species. It differs from E. brocki in having an abdomen of one segment bearing two expanded caudal rami that form a natatory appendage similar to that of Waptia fieldensis (pl. 27, figs. 4 and 5). The systematic position of the genus is doubtful. It is probably a form nearer the Merostomata than the Branchiopoda.
Formation and locality.—Middle Cambrian: Burgess shale member of the Stephen formation (about 75 feet above the phyllopod bed near the base of the shale), on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.
Order NOTOSTRACA Calman[37]
NARAOIDÆ, new family
Carapace large, with hepatic cæca in anterior portion; eyes pedunculate. Head with 5 ? pairs of appendages. Thorax with 17 to 19 segments. Abdomen with 2 to 3 segments. Thoracic appendages leg-like, with setiferous fringes and probably gills attached to the basal joints.
One genus, Naraoia.
NARAOIA, new genus
The generic description is included with that of the type species.
Genotype.—Naraoia compacta, new species.
Stratigraphic range.—The stratigraphic range is limited to a band of dark siliceous shale about 4 feet in thickness forming a part of the Burgess shale member of the Stephen formation.
Geographic distribution.—On the slope of the ridge between Wapta Peak and Mount Field, north of Burgess Pass, and about 3800 feet above Field on the line of the Canadian Pacific Railway, British Columbia, Canada.
The generic name is derived from Narao, the name of a group of small lakes in Cataract Brook canyon, above Hector on the Canadian Pacific Railway, British Columbia, Canada.
NARAOIA COMPACTA, new species
Plate 28, figs. 3 and 4
General outline of dorsal carapace elongate oval. It is divided into two subequal parts forming the cephalic carapace and a posterior or thoracic carapace. When flattened on the shale and not distorted, the two parts are subequal in size and outline. The anterior part is distorted in figure 4, but the posterior part has nearly the natural outline of a specimen when flattened out. It has a slight inward arching at the median line where the abdomen passes from beneath it. The carapace was very thin and is now frequently wrinkled and folded in a manner resembling pressed and dried specimens of the carapace of the recent Lepidurus glacialis.
The two parts of the carapace appear to be attached along the longitudinal median line to the dorsal surface of a number of the segments of the head and thorax. The line between the two parts of the carapace appears to be at about the third thoracic segment of the body. There is nothing in the appearance of the cephalic carapace to indicate how many segments are coalesced in it, but on one specimen of a posterior part 14 segments are faintly indicated. Whether these are only the impressions of the underlying segments or represent coalesced segments I am not prepared to state.
The body is slender and composed of several cephalic segments, probably 5, and 17 to 19 thoracic segments. Three of the latter appear beneath the anterior part of the carapace, 14 beneath the posterior part, and two extend beyond the posterior edge of the carapace. An abdomen is indicated by two small segments and a short, slender-jointed telson-like extension (fig. 4).
Appendages.—In the head of Burgessia bella (pl. 27, fig. 3) the cephalic appendages are all anterior to the lateral canals connecting the hepatic cæca and alimentary canal. Specimens of Naraoia compacta show the hepatic tubes, and anterior to it the outline of four divisions or segments of the central axis of the head. What may be the outer end of a simple straight antenna projects from the side of the carapace, and seventeen legs extend from beneath the carapace in figure 4. Of these, three are referred to that portion of the body beneath the anterior part and 14 to the posterior part of the carapace. The legs have long, slender joints, all of which except the distal have a strong fringe of fine setæ. The legs terminate in a minute, slightly curved claw. I have not seen a flabellum or gill in position, but considerable evidence of their presence along the side of the body is furnished by faint outlines showing through the carapace.
Interior structure.—The large hepatic cæca are beautifully shown in the sides of the anterior half of the carapace (fig. 4) also the canal connecting with the alimentary canal. The latter canal is finely shown in the thorax, where it extends to the posterior segment a little back of the posterior margin of the carapace, where the slender telson joins the body.
Observations.—This species furnishes another interesting addition to the group of Middle Cambrian Branchiopoda from the Burgess shale. It is essentially Burgessia-like (pl. 27, figs. 1-3) with the addition of the posterior half of the carapace extended back over the thorax. The hepatic cæca and legs are of the same type. Nothing is yet known of the eyes and labrum, and only a suggestion of the cephalic appendages.
Formation and locality.—Middle Cambrian: (35k) Burgess shale member of the Stephen formation, on the west slope of the ridge betweent Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.
BURGESSIDÆ, new family
Carapace forming a dorsal shield; paired eyes sessile; body with fourteen pairs of appendages of which five are cephalic, eight thoracic, and one abdominal, as now known; many segments, thirty or more, in the abdominal (telson) extension of the body. Labrum relatively large and attached to the anterior reflected edge of the carapace. Thoracic appendages leg-like and with small branchial lobes.
One genus, Burgessia.
Observations.—Anticipating that there will be many more specimens available for study in the near future, I will not attempt to correlate the Burgessidæ with any described faimly of the Notostraca. I suspect that Burgessia bella is the representative of an irregular order that, like the Leptostraca, does not fall strictly within the definition of the subclass to which it is referred.
BURGESSIA, new genus
The generic description is included with that of the type species.
Genotype.—Burgessia bella, new species.
Stratigraphic range.—The stratigraphic range is limited to a band of dark siliceous shale about 4 feet in thickness forming a part of the Burgess shale member of the Stephen formation.
Geographic distribution.—On the slope of the ridge between Wapta Peak and Mount Field, north of Burgess Pass, and about 3800 feet above Field on the line of the Canadian Pacific Railway, British Columbia.
The generic name is derived from Burgess, the name of a mountain and pass near the fossil bed from which Burgessia bella was collected, British Columbia, Canada.
BURGESSIA BELLA, new species
Plate 27, figs. 1–3, and plate 30, figs. 3 and 4
Carapace large, semicircular in outline when pressed flat, with a rounded notch at the posterior side where the thorax appears from beneath the carapace. The test is very thin and it has often been wrinkled and distorted in the shale. Ten specimens show it pressed down sideways so as to give the outline of a bivalve carapace (figs. 3 and 4, pl. 30).
The thorax is slender and composed of 8 rather short segments that bear appendages.
The abdomen is composed of one segment that is about the size of the last thoracic segment; it is followed by a long, slender, many-jointed telson-like extension that tapers gradually to a very fine, thread-like extremity. An abdomen of one segment has a telson 21 mm. in length attached to a body 12 mm. long, and composed of over thirty segments.
Eyes.—The paired eyes are shown on three specimens. In one a slight convexity still remains and in all a minute, round, bright dot indicates the eye a short distance within the anterior margin of the carapace.
Labrum.—A narrow labrum is outlined in a number of specimens between what appear to be two branches of the alimentary canal. In figure 1, plate 27, the labrum is on the under side and only the broad anterior end (stomach) of the alimentary canal is shown. In figures 2 and 3, plate 27, the labrum lies over the stomach and causes it to appear forked. It is shown more definitely in other specimens.
Appendages.—Many specimens have two slender, short-jointed antennæ projecting out from in front of the carapace (fig. 2, pl. 27). Others show a second shorter, smaller pair that is nearer the median line and probably represents the antennules. Several specimens have two or three very slender jointed appendages projecting from beneath the carapace posterior to the antenna. A flattened specimen of the under side of the head shows the basal joints of the first five pairs of appendages (fig. 3, pl. 27). An antenna may be traced to the second joint. The third shows only an obscure inner joint; the fourth has two long slender joints (1 and 2) and the fifth two rather broad joints. (Traces of the cephalic appendages are shown by fig. 2, pl. 27.) Where what may be the terminal joints of the third to fifth appendages project beyond the carapace, they are very delicate, slender, and one at least ends in two fine filaments.
The first five pairs of appendages are in front of the large tubes (cl, fig. 1) coming in from each side.
The thoracic legs have at least seven joints, the last pointed and curved slightly with a delicate terminal spine or claw. The three inner joints are larger than the outer and have a flattened triangular expansion of the inner side that gives a nodelike appearance to the leg when flattened so as to bring this feature in profile. These triangular expansions also show on the fourth and fifth joints of some specimens. One specimen shows on seven pairs of legs, small, elongate, oval bodies attached near the first joint to the outer side of the leg. These bodies left but slight impression on the rock and are rarely seen. They appear to represent the gills.
A pair of minute, jointed, setiferous appendages projecting from beneath the first abdominal segment suggests the presence of a simple phyllopodan natatory leg. The remaining thirty or more segments of the abdomen and telson are limbless so far as can be determined from many specimens.
Interior structure.—The thin carapace has preserved and now shows most beautifully the large hepatic cæca. The position and connection of these is finely shown at (kd) by figures 1 and 2, plate 27. The alimentary canal is large, expanded in the head as a stomach (st, fig. 1), and extending directly through the body from the front of the head to the first abdominal segment where it presumably terminated at the anus.
Dimensions.—The average length of the larger specimens of the carapace is about 10 mm. Some are 12 mm. and many 6 to 8 mm. The proportions of carapace, thorax, and abdomen are fairly well shown by figure 1, except that the long, thin abdomen continues backward until it exceeds the entire length of head and thorax about 3 to 2, or by actual measurement in one example, 21 mm. to 12 mm. for the head and body respectively.
Observations.—The very delicate carapace resembles that of the recent Lepidurus and like the latter takes many forms when flattened by pressure. An illustration of the deformation of the carapace of Burgessia is given by figures 3 and 4, plate 30. I at first thought that the latter represented quite a different form from Burgessia bella, but with the examination of many specimens a fine series was selected, showing gradations between the typical specimens on plate 27, figures 1 and 2, and the crushed side views shown by figures 3 and 4, plate 30. I had selected many specimens to be photographed but decided to illustrate only five in this preliminary paper as many interesting points have come up that more material may throw light on.
Among living Branchiopoda the Apodidæ furnish the most suggestive examples for comparison with Burgessia bella. The absence of abdominal segments with appendages is a marked distinction, also the presence of eight pairs of thoracic legs. The long slender abdominal section points to the disappearance of appendages such as occur in the Apodidæ and the diminution in size of the abdominal segments and probably to the ultimate disappearance of most of them.
Formation and locality.—Middle Cambrian: (35k) Burgess shale member of the Stephen formation, on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.
Family (undetermined)
Genus ANOMALOCARIS Whiteaves
ANOMALOCARIS GIGANTEA, new species
Plate 34, fig. 3
Practically all that is known of this species is illustrated by figure 3 of plate 34 (natural size), which gives a side view of the abdomen of the species. Nothing is known of the carapace or of the details of the appendages more than that for each segment there are two strong pointed appendages that appear to be composed of two joints; the long, narrow, sharp distal joint, and a short, broad proximal joint.
One specimen found in association with the other fragments indicates, if it belongs to the same species, that the abdomen terminated in a short, strong, slightly curved telson.
This species differs from Anomalocaris canadensis Whiteaves[38] in its greater size and more compact abdominal segments.
It is hoped that more perfect material will be found at the Burgess Pass locality that will enable us more clearly to determine this species, also to discover the nature of its carapace and that of the other described species of Anomalocaris which occur on the slope of Mount Stephen at nearly the same horizon about six miles away.
Formation and locality.—Middle Cambrian: (35k) Burgess shale member of the Stephen formation (phyllopod bed), on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field. British Columbia.
WAPTIDÆ, new family
A transition form between the Branchiopoda and Malacostraca with a small carapace covering more or less of the cephalic and thoracic region. Abdominal region with 6 segments the last of which bears a pair of fin-like rami or a slender telson.
Thorax with 8 segments bearing more or less foliaceous jointed appendages that carry a small scale-like exopodite, or it may be an epipodite.
Eyes pedunculated.
One genus, Waptia, is referred to this family.
WAPTIA, new genus
The generic description is included with that of the type species.
Genotype.—Waptia fieldensis, new species.
Stratigraphic range.—The stratigraphic range is limited to a band of dark siliceous shale about 4 feet in thickness forming a part of the Burgess shale member of the Stephen formation.
Geographic distribution.—On the slope of the ridge between Wapta Peak and Mount Field, north of Burgess Pass, and about 3800 feet above Field on the line of the Canadian Pacific Railway, British Columbia, Canada.
Generic name derived from Mount Wapta, a mountain above the fossil bed in which the type specimens of the genus and species were found.
WAPTIA FIELDENSIS, new species
Plate 27, figs. 4 and 5
Carapace about one-third the length of the body. Seen from the side it is broadly oval in outline with the upper side slightly curved. From above, when flattened out, it is narrowed toward the front and projects into broad lobes separated by a forward curving toward the median line. In figure 5 the carapace has been pushed forward and turned over so as nearly to reverse its true position. In figure 4 it has been compressed laterally so as to give the posterior parts a wing-like appearance. The test was so delicate that only a few specimens show even approximately the original outlines.
Body long and slender. It is divided into a head, trunk or thorax, abdomen, and tail.
The head cannot be clearly described as it is largely concealed by the carapace. The presence of appendages indicates that 5 segments are combined to form it.
The trunk or body is formed of eight short segments, nearly equal in size, each bearing a pair of appendages.
The abdomen has six long, sharply defined segments with short spines on the posterior margin. The caudal rami are expanded into rather broad lobes that overlap slightly so as to form a strong caudal fin.
The eyes are situated on each side just beyond the antennæ (fig. 4). When pressed flat they show a crescentiform outline at the end of a short strong peduncle.
Appendages.—The antennæ are long and slender with rather long joints. Between the antennæ in one specimen a pair of short lobe-like appendages occur that for the present I shall consider as the antennules. They are not shown in the specimens illustrated. The first pair of leg-like appendages seen show six joints to where they are lost beneath the carapace (m, fig. 5). The next posterior four have fine curved spines on the terminal joint with short joints carrying strong setæ on their back margin. The posterior six pairs have all the joints heavily fringed with setæ and the terminal joint apparently has two or more narrow, elongate, lobe-like prolongations. Pressed down on the basal joint there is a lance-shaped, short, flat lobe that may be the exopodite or possibly the epipodite. The large, broad, setiferous, outer joints of the six posterior pairs of legs were undoubtedly natatory in their action, and the basipodite also probably carried branchiæ on the epipodite. On one specimen such lobes are shown on three of the legs. No traces of any appendages have been seen on the posterior six segments of the body.
Alimentary canal.—This may be traced as a small, straight canal from the head back to the point where the caudal rami unite with the posterior segment.
Dimensions.—The largest specimen in the collection has a length of nearly 65 mm. Its proportions, in side view, are shown by figure 5, plate 27.
Observations.—This is one of the most beautiful and graceful of the remarkable group of crustaceans from the Burgess shale. It occurs in relative abundance but unfortunately I have not yet found a specimen showing clearly the arrangement of the various appendages beneath the anterior portion of the body.
Formation and locality.—Middle Cambrian: (35k) Burgess shale member of the Stephen formation, on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.
Sub-Class MALACOSTRACA
Order HYMENOCARINA Clarke
Family HYMENOCARIDÆ Salter
Genus HYMENOCARIS Salter
- Hymenocaris Salter, 1853, Rept. British Assoc. Adv. Sci. for 1852. On the Lowest Fossiliferous Beds of North Wales, p. 58. (Genus briefly described. Genotype = Hymenocaris vermicauda Salter.)
- Hymenocaris Salter, 1866, Mem. Geol. Survey Great Britain, Vol. 3, p. 293, pl. 2, figs. 1-4, pl. 5, fig. 25. (Description of genus slightly changed and illustrations given of type species.)
- Hymenocaris Etheridge, 1881, Mem. Geol. Survey Great Britain, Vol. 3, 2d ed., p. 484. (Reprint of description by Salter, 1866.)
The generic description by Salter (1866) is as follows:
Carapace ample, semi-oval, narrowed towards the front, curved downward at the sides, but not angularly bent along the dorsal line; no external eyes; antennæ?; abdomen as long or longer than the carapace, of nine transverse segments, the last with three pairs of unequal lanceolate appendages.
The illustrations accompanying the description by Salter show the general form of the carapace and abdomen. These taken in connection with seven specimens of the carapace, two of which have several segments of the abdomen attached to them (in the collection of the United States National Museum), enable me to identify the genus and add materially to the description of Salter.
On one of the specimens of the carapace [Salter, 1866, pl. 2, fig. 3] two antennæ are shown, otherwise no traces of the appendages of the head or body are mentioned.
Hymenocaris perfecta (pl. 31, fig. 2) shows the antennæ to be jointed, while the antennæ noted by Salter for Hymenocaris vermicauda were unjointed.
The genus and its type species have been referred to by authors many times during the past fifty years, and Salter's diagrammatic figure has been copied into nearly all text-books in which the fossils of the Cambrian system are illustrated.
In addition to those described in this paper there are a number of American species of Hymenocaris known. These include H. argentea (Walcott)[39] from the Middle Cambrian of Utah, and several undescribed species from the Middle Cambrian of the Cordilleran province of western North America.
The valves of the carapace of Isoxys acutangula[40] (Walcott) are abundant in the lower portion of the Burgess shale, and there are also fragments of the carapace of a very large form that possibly may be related to Hurdia victoria (pl. 32, fig. 9).
HYMENOCARIS PERFECTA, new species
Plate 31, figs. 1-6 (upper specimen)
The form and outline of the carapace are shown as flattened on the shale by figure 1 on side view and somewhat roughly from dorsal view by figure 2. Several specimens show seven abdominal segments extending beyond the carapace. The terminal segment has from two (fig. 4) to six (fig. 5) cercopods attached to it.
A strong adductor muscle scar (adm, figs. 1 and 2) is shown on many specimens.
A pair of small pedunculated eyes project in front of the carapace, one showing on each side of a pair of minute antennules.
Appendages.—Head. Several specimens show a pair of minute, jointed antennules projecting forward from between the large jointed antennæ (fig. 2). The antennæ are large and are composed of a single stem of short joints; they may, however, be straight, unjointed, and long (fig. 2, pl. 31). I have not illustrated a specimen showing the antennules and eyes, as they were not observed until after the plates were made up.
The specimen illustrating the thoracic legs and head appendages (fig. 1) is unfortunately not so good as one which was found and cleaned of calcareous deposits after the plates were finished and before this description was written. This specimen shows three cephalic legs. The two anterior are slender (mandible and maxillula), and the posterior maxilla is large and formed of short strong joints. There are eight pairs of thoracic legs. The distal portions of these are finely shown in figure 1. The broad, setiferous joints of the exopodite are also shown near the carapace. In other specimens they extend out over the legs so as nearly to conceal them. Traces of oval gills (epipodites) are shown for three legs on the outer side of what appears to be the second joint of the leg.
Interior structure.—The alimentary canal may be traced from the anterior part of the body back to the posterior abdominal segment where it terminates between two larger cercopods of the type represented by figures 1 and 2. One specimen, not illustrated, appears to have a considerable enlargement of the canal in the head portion.
Dimensions.—The valves of the carapace average from 40 to 60 mm. in length, with other proportions as shown by figure 1.
Formation and locality.—Middle Cambrian: (35k) Extending through about 25 feet of the Burgess shale member of the Stephen formation, on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.
HYMENOCARIS ? CIRCULARIS, new species
Plate 32, fig. 4
This is a much smaller species than the other species of this genus from the Burgess shale and I am not sure that it should be referred to the genus. All that is known of its appendages is shown by figure 4. A number of specimens of the valves in the collection average 5 to 8 mm. in length.
Formation and locality.—Middle Cambrian: (35k) Burgess shale member of the Stephen formation, on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.
HYMENOCARIS OBLIQUA, new species
Plate 32, figs. 1-3
This form differs in the form of the carapace from H. perfecta, as may be seen by comparing figures 1-3, plate 32, with figure 1, plate 31.
Formation and locality.—Middle Cambrian: (35k) Burgess shale member of the Stephen formation, on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.
HYMENOCARIS OVALIS, new species
Plate 32, figs. 5 and 6
The outline of a valve of the carapace is illustrated by figure 5, which is 15 mm. in length. A laterally compressed carapace and abdomen are represented by figure 6. These two figures illustrate all that is known of the species.
Formation amd locality.—Middle Cambrian: (35k) Burgess shale member of the Stephen formation, on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.
HYMENOCARIS ? PARVA, new species
Plate 32, fig. 7
This small species is represented by two specimens. At first it was placed with H. ? circularis (pl. 32, fig. 4), but later it was found to differ in its appendages. The specimen illustrated by figure 7 has the carapace crushed down so as to appear broad oval in outline, but another specimen has a nearly straight dorsal margin. The abdomen is pushed over on to the carapace. The antennæ project from the left side.
Formation and locality.—Middle Cambrian: (35k) Burgess shale member of the Stephen formation, on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.
Family (undetermined)
HURDIA, new genus
The generic description is included with that of the type species.
Genotype.—Hurdia victoria, new species.
Stratigraphic range.—The stratigraphic range is limited to a band of dark siliceous shale about 4 feet in thickness forming a part of the Burgess shale member of the Stephen formation.
Geographic distribution.—On the slope of the ridge between Wapta Peak and Mount Field, north of Burgess Pass, and about 3800 feet above Field on the line of the Canadian Pacific Railway, British Columbia, Canada.
The generic name is derived from Hurd, the name of a mountain northeast of Leanchoil on the Canadian Pacific Railway, British Columbia, Canada.
HURDIA VICTORIA, new species
Plate 32, fig. 9
Of this species only the valves of the carapace are known. The illustration (pl. 32. fig. 9) shows the natural size and proportion of a right valve. A larger specimen has a length of 13.5 cm.
The test was quite thin and readily compressed and distorted, which causes considerable variation in the outlines of the valve.
A faintly outlined reticulation of the surface is shown on several specimens.
The only nearly related form known to me is Hurdia triangulata.
Formation and locality.—Middle Cambrian: (35k) Burgess shale member of the Stephen formation, on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.
HURDIA TRIANGULATA, new species
Plate 34, fig. 1
The left valve illustrating this species is slightly distorted by compression, but it outlines the average form. The largest specimen of a single valve in the collection has a length of about 10 cm. with a depth of 6 cm.
This species differs from Hurdia victoria in having a valve proportionately shorter and deeper.
Formation and locality.—Middle Cambrian: (35k) Burgess shale member of the Stephen formation, on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.
TUZOIA, new genus
The generic description is included with that of the type species.
Genotype.—Tuzoia retifera, new species.
Stratigraphic range.—The stratigraphic range is limited to a band of dark siliceous shale about 4 feet in thickness forming a part of the Burgess shale member of the Stephen formation.
Geographic distribution.—On the slope of the ridge between Wapta Peak and Mount Field, north of Burgess Pass, and about 3800 feet above Field on the line of the Canadian Pacific Railway, British Columbia, Canada.
The generic name is derived from Tuzo, the name of one of the mountains of the "Valley of the Ten Peaks" south of Laggan, Alberta, Canada.
TUZOIA RETIFERA, new species
Plate 33, fig. 2
Of this genus and species only one specimen is known. Its large size, form, and reticulated surface serve to distinguish it from all other known forms. The figure on plate 33 shows very clearly that the test was thin, as it has been crowded and wrinkled near the longitudinal center.
The reticulated surface marking is not unlike that of the carapace of the recent Nebaliopsis typica Sars.[41]
Formation and locality.—Middle Cambrian: (35k) Burgess shale member of the Stephen formation (phyllopod bed), on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.
ODARAIA, new genus
The generic description is included with that of the type species.
Genotype.—Odaraia alata, new species.
Stratigraphic range.—The stratigraphic range is limited to a band of dark siliceous shale about 4 feet in thickness forming a part of the Burgess shale member of the Stephen formation.
Geographic distribution.—On the slope of the ridge between Wapta Peak and Mount Field, north of Burgess Pass, and about 3800 feet above Field on the line of the Canadian Pacific Railway, British Columbia, Canada.
The generic name is derived from Odaray, the name of a peak west of Lake O'Hara and south of Hector, on the Canadian Pacific Railway, British Columbia, Canada.
ODARAIA ALATA, new species
Plate 34, fig. 2
Several specimens of the valves of this large fine species occur in the collection, but unfortunately all of them are more or less crushed and distorted. The one illustrated on plate 34 (natural size) indicates that the test of the carapace was very thin and readily wrinkled and broken. This specimen has, projecting from under the posterior margin of the valve, portions of three of the posterior segments of the abdomen with one of the large cercopods attached and one crowded under and out of place.
There is no probability of this genus or species being confused with any described form.
Formation and locality.—Middle Cambrian: (35k) Burgess shale member of the Stephen formation (phyllopod bed), on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.
FIELDIA, new genus
The generic description is included with that of the type species.
Genotype.—Fieldia lanceolata, new species.
Stratigraphic range.—The stratigraphic range is limited to a layer in a band of dark siliceous shale about 4 feet in thickness forming a part of the Burgess shale member of the Stephen formation.
Geographic distribution.—On the slope of the ridge between Wapta Peak and Mount Field, north of Burgess Pass, and about 3800 feet above Field on the line of the Canadian Pacific Railway, British Columbia, Canada.
The generic name Fieldia is derived from Field, the name of a mountain rising above Burgess Pass northeast of Field, British Columbia, Canada.
FIELDIA LANCEOLATA, new species
Plate 32, fig. 8
This species is so distinctly characterized by the long slender form of its valves that it is not apt to be confused with any other species. It is further characterized by five narrow longitudinal bands.
The numerous small appendages projecting outside of the carapace on the lower side indicate that the body had many segments and appendages, but with only one specimen for study I shall not attempt at this time to discuss it, as it is possible that other specimens will be discovered during the field season of 1912.
Formation and locality.—Middle Cambrian: (35k) Burgess shale member of the Stephen formation, on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.
CARNARVONIA, new genus
The generic description is included with that of the type species.
Genotype.—Carnarvonia venosa, new species.
Stratigraphic range.—The stratigraphic range is limited to a band of gray, buff-weathering siliceous shale forming the upper part of the Burgess shale member of the Stephen formation.
Geographic distribution.—On the slope of the ridge between Wapta Peak and Mount Field, north of Burgess Pass, and about 3800 feet above Field on the line of the Canadian Pacific Railway, British Columbia, Canada.
The generic name Carnarvonia, is derived from Carnarvon, the name of a mountain of the President Range, northwest of Field, British Columbia, Canada.
CARNARVONIA VENOSA, new species
Plate 33, fig. 1
The figure on plate 33, of the two valves united and pressed flat on the shale, illustrates all that is known of this genus and species. The size and proportions are shown by the figure.
The reticulated surface, adductor muscle scars, and vascular markings on the shell are beautifully shown on the specimen and in this illustration.
Formation and locality.—Middle Cambrian: Burgess shale member of the Stephen formation (about 75 feet (22.8 m.) above the phyllopod bed near the base of the shale), on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.
Sub-Class TRILOBITA
NOTES ON SOME APPENDAGES OF THE GENERA NEOLENUS AND PTYCHOPARIA
We now have from the Burgess shale specimens of a Middle Cambrian trilobite (Neolenus serratus (Rominger)), showing the character of its antennæ, legs, branchiæ, and caudal appendages, and another (Ptychoparia cordilleræ (Rominger)) with a full series of branchial appendages. Also there are five Ordovician forms, three from the Trenton limestone (Calymene senaria, Ceraurus pleurexanthemus, and Asaphus platycephalus) and two from the Utica shale (Triarthrus becki and Trinucleus concentricus) , which preserve the antennæ, legs, and branchiæ.
A review of these shows a surprising uniformity of structure of the antennæ, legs, and branchiæ in genera separated by great intervals in the stratigraphic series, and distinguished by marked variations in the external form of the dorsal shield.
At first[42] I was inclined to consider that the trilobite was a highly organized crustacean approaching the merostomes, but with the data now available I join with Burmeister[43] and Bernard[44] in considering that the trilobite is more closely related to the branchiopod crustaceans. Burmeister wrote in 1843:[45]
The trilobites were a peculiar family of Crustacea, nearly allied to the existing Phyllopoda, approaching this latter family most nearly in its genus Branchipus, and forming a link connecting the Phyllopoda with the Pœcilopoda.
In order, however, to estimate fairly the affinity of the trilobites with the Phyllopoda, we must not lose sight of the important fact that the trilobites differ not only from the Phyllopoda, but from all other existing families of Crustacea in the varying numerical proportion of their thoracic rings; a peculiarity neither exhibited at present as a characteristic of any natural family among the Crustacea, nor in any of the heterogeneous Articulata. This peculiarity occurs, it is true, among the Aspidostraca (a group of the second great division of the crustaceans), but only in a modified form, the difference in the numerical proportion being always reducible to one fundamental number. This law is apparently not observed in the case of the trilobites.
It would seem then that the relation existing between the trilobites and the existing Crustacea is one rather of analogy than affinity, so that the whole group may be considered as a separate division, corresponding with the Aspidostraca in the formal variation presented from the typical character, but not to be looked on as a nearly allied or similar group to this or to other tribes.
Putting out of the question the important difference exhibited in the numerical proportion of the thoracic rings just alluded to, this analogy to the Aspidostraca might certainly have been considered as very close—all the other relations of organization, so far as they can be traced, corresponding very accurately—if it were not for the structure of the extremities. These, indeed, which are hard, horny, and articulated in a sub-division of the present Aspidostraca, were probably entirely absent in this form in Trilobites; but in other respects all the typical characters of the two groups will be found to correspond.
Bernard[46] concluded that—
Apus, on account of its richer segmentation, the absence of pleuræ on the trunk-segments, and its more membraneous parapodia-like limbs, must be assumed to lie in the direct line upwards from the original annelidian ancestor toward the modern crustacea. The trilobites then must have branched off laterally from this line either once or more than once, in times anterior to the primitive Apus, as forms specialized for creeping under the protection of a hard imbricated carapace.
In 1895, with the new evidence afforded by the trilobite Triarthrus becki, he concluded[47] that—
The trilobites, therefore (as exemplified by Triarthrus), in spite of their extremely primitive mouth-formula, do not stand in the direct line of descent of the Crustacea, but are lateral offshoots, specialized for a creeping manner of life.
The discovery of caudal rami on Neolenus (pl. 24, figs. 1 and 1a) still further accentuates the conclusion of Bernard that the trilobites were derived from the same stock as Apus. This is further strengthened by the presence in the Middle Cambrian of a form like Nathorstia transitans (pl. 28, fig. 2), which is essentially a trilobite, but its setiferous thoracic appendages relate it closely to Opabinia regalis (pl. 27, fig. 6).
Neolenus serratus (Rominger).— A number of specimens of this species show the antennæ, jointed thoracic legs, and caudal rami. One of the specimens is illustrated by figure 1, plate 24. In this the caudal rami have been displaced and dragged back, bringing a portion of the ventral surface of the abdomen. Figure 1a shows the caudal rami in their normal position. I have already illustrated the filamentous branchial thoracic appendage of this species.[48] The resemblance between these branchiæ and the branchiæ or gills of the branchiopod Waptia fieldensis (pl. 27, figs. 4 and 5) is very striking.
Ptychoparia cordilleræ (Rominger).—A small specimen, with the dorsal shield exfoliated (pl. 24, fig. 2), shows an antenna and a long series of the setiferous or filamentous exopodites. A specimen recently worked out shows narrow, elongate, fringe-like appendages attached to the setiferous branchial appendages that are similar in appearance to the abdominal branchial appendages of Sidneyia.[49]
In the near future I wish to review the conclusions published in my paper of 1881,[50] and those that have been entertained regarding Triarthrus becki and the new material from the Burgess shale.
DESCRIPTIONS OF NEW GENERA AND SPECIES
Order (undetermined)
MARRELLIDÆ, new family
Carapace strong, small, subquadrangular and with two postero-lateral spines comparable with the lateral lobes of the carapace of Apodidæ. Eyes sessile. Head with five pairs of appendages. Thorax with 24 pairs of appendages. Abdomen a single plate-like telson. Thoracic leg with jointed leg-like endopodite, a jointed setiferous exopodite, and expanded gill-like epipodite.
One genus, Marrella.
Observations.—This family is instituted to include Marrella splendens, a species that, despite its remarkable carapace and cephalic appendages, recalls Apus and Lepidurus. It differs from the two latter so markedly in its carapace and abdomen that it becomes the type of a family that is less primitive than the Apodidæ and may be considered as near the Trilobita (p. 162).
MARRELLA, new genus
The generic description is included with that of the species.
Genotype.—Marrella splendens, new species.
Stratigraphic range.—The stratigraphic range is limited to a band of dark siliceous shale about 4 feet in thickness forming a part of the Burgess shale member of the Stephen formation.
Geographic distribution.—On the slope of the ridge between Wapta Peak and Mount Field, north of Burgess Pass, and about 3800 feet above Field on the line of the Canadian Pacific Railway, British Columbia, Canada.
The generic name is given in recognition of the geologic and paleontologic work of my friend. Dr. John E. Marr, Johns College, Cambridge, England.
MARRELLA SPLENDENS, new species
Plate 25, figs. 1-6, and plate 26, figs. 1-6
The general form is shown by figures 1, 4, and 5, plate 26. The exoskeleton is composed of a strong cephalic carapace (c) (fig. 1, pl. 26) which extends as two long, strong, curved spines (x) that continue posteriorly over the back of the thorax beyond the end of the body. At each antero-lateral angle a strong, backward-curving spine (a') complements the great dorsal thoracic spines. A pair of large crescentiform sessile eyes occur on the anterior margin just within the base of the anterior spines (pl. 25, figs. 4 and 5). The great dorsal spines are beautifully crenulated on the outer margin by short, strong backward-curving spines.
Appendages.—The antennæ (a", fig. 5, pl. 26) are long, slender, and many-jointed; they unite with the head near the posterior end of the labrum; the third pair of appendages, mandibles (m), are large, long, 7 ?-jointed and with fine setæ on the edge of the joints which give the appearance of a slender feather to the appendage (fig. 3, pl. 25, and fig. 3, pl. 26). The fourth and fifth cephalic appendages are slender, and with long joints.
There are 24 pairs of thoracic appendages. Each one is composed of a jointed leg of seven joints with a flattened, short, broad spine on all but the proximal and distal joints (thl, fig. 6, pl. 26); ten legs, (endopodites) with the expanded joints are shown in figure 6, plate 26; anterior to these there are preserved 4 setiferous appendages that appear to be the exopodites of the leg. These are more fully shown by figure 6, plate 25, also figure 3. Another view of the long, jointed endopodite is found in figures 3 and 5, plate 26. The presence of a gill (epipodite) was unsuspected until the specimen represented by figure 4, plate 26, was found; this fine example is so delicate and so beautifully preserved that it is almost unique even among the wonderful Burgess shale fossils.
Abdomen.—The abdomen (ab, figs. 1, 3, 4, and 6, pl. 26) forms a small, plate-like termination of the long body.
Interior structure.—The alimentary canal is very distinct in a number of specimens (i, figs. 3 and 6, pl. 26). It extends from the posterior margin of the labrum back to the plate-like abdomen. The head is too much smashed down to show any details of interior structure, but the alimentary canal appears to widen out and occupy much of the space beneath the subquadrangular carapace. The large dorsal spines have a central canal that appears to open into the space (stomach ?) beneath the carapace; this canal may represent the hepatic cæca of Burgessia (pl. 27, figs. 1-3). The slender canal of the antennules joins the visceral space beneath the carapace at its antero-lateral angle.
Dimensions.—The average length is 15 mm. The size, proportions and relations of parts are shown by the figures on plates 25 and 26. The animal was so delicate that it was readily smashed and distorted.
Observations.—This beautiful fossil (it was called the "Lace crab" at camp) is the most abundant of the many species in the phyllopod bed. It must have swarmed in large numbers in the quiet waters.
Reference to its relations to other crustaceans will be found in the introduction.
Formation and locality.—Middle Cambrian: (35k) Burgess shale member of the Stephen formation, on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.
Family (undetermined)
NATHORSTIA, new genus
The generic description is included with that of the type species.
Genotype.—Nathorstia transitans, new species.
Stratigraphic range.—The stratigraphic range is limited to a layer in a band of dark siliceous shale about 4 feet in thickness forming a part of the Burgess shale member of the Stephen formation.
Geographic distribution.—On the slope of the ridge between Wapta Peak and Mount Field, north of Burgess Pass, and about 3800 feet above Field on the line of the Canadian Pacific Railway, British Columbia, Canada.
The generic name is proposed in honor of Dr. Alfred G. Nathorst, the distinguished Swedish paleontologist and paleobotanist.
NATHORSTIA TRANSITANS, new species
Plate 28, fig. 2
Dorsal shield elongate-oval in outline, very thin and delicate in structure. It may be divided into a cephalic region (cephalon), thorax, and abdomen. The cephalon is transversely semicircular with a short spine at each posterolateral angle; obscurely trilobed. One of the crescent-shaped, medium-sized eyes (e) is indicated on the right of the central axis.
Thorax faintly trilobed, composed of eight rather long (longitudinal) segments. The specimen illustrated is so flattened and crushed that it is difficult to determine the form of the segments, but other specimens with several segments united show them to be much like those of Molaria spinifera (pl. 29, fig. 2).
The abdomen (pygidium) is apparently semicircular with a rudely defined median lobe and two or three segments outlined on it.
Appendages.—Head. A portion of what may be an antenna projects from beneath the right anterior margin; from near the left posterolateral angle a large four-jointed appendage extends backward. I assume that this may be the outer portion of the large posterior appendage (maxilla) of the head.
Thorax. Traces of several slender-jointed thoracic legs project from beneath the anterior segments and back of these on the right side more or less of six legs have been pushed out from beneath the dorsal shield; these are composed of three to four long, slender joints; fragments of the three proximal joints indicate that they are shorter and larger and that they have a fringe of fine setæ. Indications of a branchial lobe (gill) are seen in two specimens where the legs are not preserved. This is often the case both among the Merostomata (pl. 29, fig. 3) and Trilobita (pl. 24, fig. 2).
Two caudal rami project a little distance from beneath the posterior margin of the dorsal shield.
Dimensions.—The only entire specimen has a length of 45 mm. Its other proportions are shown by figure 2, plate 28.
Observations.—I have given the specific name transitans to this species on account of its suggesting a transition between a Merostome-like form, such as Molaria spinifera, and the trilobites (pl. 24, figs. 1 and 2). This is mentioned under remarks on the appendages of the trilobites (p. 191).
The specimen illustrated was found by my son Sidney where we were using dynamite to "sledge" our way into the solid ledge of hard shale as it was back from the action of frost that the most beautifully preserved specimens were found. A few fragments turned up later, and we hope to find more perfect specimens in the future.
Formation and locality.—Middle Cambrian: (35k) Burgess shale member of the Stephen formation (phyllopod bed), on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.
Order HYPOPARIA Beecher
Family (undetermined)
It is highly probable that the new genera Mollisonia and Tontoia will come within the family Microdiscidæ Coquin, 1896.
MOLLISONIA, new genus
General outline elongate, sides subparallel; cephalon and pygidium subequal in size and outline, and indistinctly lobate as now known. Eyes unknown. Thorax with seven simple transverse segments that may or may not be divided into axial and lateral longitudinal lobes by slight depressions on the line of each third of the segment. Surface finely granular.
Genotype.—Mollisonia symmetrica, new species.
Stratigraphic range.—Middle Cambrian: Burgess shale member of the Stephen formation, British Columbia.
The generic name is derived from Mollison, the name of a mountain southwest of Field on the Canadian Pacific Railway, British Columbia, Canada.
Observations.—In its subequal cephalon and pygidium this genus resembles Agnostus and Microdiscus, but it differs in the absence of distinct lobes and the greater number of thoracic segments. The almost unsegmented cephalon and pygidium and few simple thoracic segments are characters of the most highly developed families of the Trilobites, such as Asaphidæ and Illaenidæ, but in the absence of satisfactory evidence of the presence of eyes on the cephalon further study of better material is needed. The form of the thorax recalls that of Bohemilla stupenda Barrande.[51]
MOLLISONIA SYMMETRICA, new species
Plate 24, fig. 3
General outline elongate, length about three times the width; sides subparallel; cephalon and pygidium nearly equal in size and contour; thorax with seven segments. Test thin with a minutely granular surface.
Cephalon a little shorter than its width at the posterior margin; sides nearly straight and sloping slightly inward toward the broadly rounded antero-lateral angles and front margin. The presence of eyes is very doubtfully suggested by small, faint, crescentiform depressions about 2 mm. from the antero-lateral margin. A raised line that possibly may be the facial suture extends back to the margin midway of the length of the cephalon; in front of the possible eye lobe it appears to pass across the front parallel to the margin and about 1.5 mm. from it. There are faint traces of transverse furrows indicating the presence of about five transverse lobes on the central portion of the cephalon.
The thorax has seven transverse segments outlined, the anterior of which is somewhat narrower than the others; the segments terminate in blunt, falcate, slightly furrowed ends that overlap on the next posterior segment within the posterior curve of the free portion of the end of the segment. A lobation of the thorax is indicated by the presence of a shallow, elongate depression on seven of the segments one-third of the distance across from the left side; if similar depressions existed on the right side they have been obscured by the flattening of the test in the shale.
The pygidium curves outward from its anterior margin, and the rounded outline extends along the sides and posterior margin; it shows traces of an anterior transverse furrow indicating a segment.
| Dimensions. | mm. |
| Length of dorsal shield | 48.0 |
| Width of cephalon | 17.0 |
| Length of cephalon | 13.5 |
| Greatest width of thorax | 18.0 |
| Length of thorax | 24.0 |
| Width of pygidium | 15.0 |
| Length of pygidium | 12.5 |
Observations.—The only known specimen of this unique fossil was found on a large slab of calcareous shale in association with Bathyuriscus rotundatus (Rominger), Ogygopsis klotzi (Rominger), and Anomalocaris canadensis Whiteaves. The impression of the thin test is very clear and along the left side some of the test remains as a very thin dark scale. The specimen is in the same condition of preservation as the thin tests of the shields and body of Anomalocaris canadensis and was evidently much thinner and more delicate than the tests of the associated trilobites.
Formation and locality.—Middle Cambrian: (14s) Lower portion of the Stephen formation, northwest slope of Mount Stephen, above Field, British Columbia, Canada.
MOLLISONIA GRACILIS, new species
Plate 24, fig. 5
Of this species only one specimen is known. The general outline and proportions of the dorsal shield are shown in figure 5. The specimen has been laterally compressed so that the lateral edges of the cephalon, segments, and pygidium are wrinkled and suggest fine spines.
One of the striking peculiarities is the transverse anterior merging of the cephalon into the short blunt spines projecting from it.
There are no indications of eyes on the cephalon. A narrow median longitudinal ridge occurs on the posterior half of each of the seven segments of the thorax and on an eighth segment that appears to be still attached to the pygidium.
This little species, 16 mm. in length, appears to be generically identical with Mollisonia symmetrica, which occurs at about the same geological horizon a few miles distant. It is much smaller and more slender than that species.
Formation and locality.—Middle Cambrian: (35k) Burgess shale member of the Stephen formation (phyllopod bed), on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.
MOLLISONIA ? RARA, new species
Plate 24, figs. 6 and 7
Of this species there are several fragmentary specimens. The species differs from M. gracilis, with which it is associated, in the character of the thoracic segments and pygidium; also, so far as we can determine from this superficial study, there are seven segments and the pygidium shows distinct segmentation with a denticulated border.
The specimen illustrated by fig. 6 indicates that the species was the largest of the genus and may have had a length of from 5 to 6 cm.
Formation and locality.—Middle Cambrian: (35k) Burgess shale member of the Stephen formation (phyllopod bed), on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.
TONTOIA, new genus
The generic description is included with that of the type species.
Genotype.—Tontoia kwaguntensis, new species.
Stratigraphic range.—The stratigraphic range is limited to the upper portion of the Tonto sandstone on the surface of a few thin layers of sandstone.
Geographic distribution.—The species has been found only in Kwagunt Valley off the eastern end of Kiabab Plateau in the Grand Canyon of the Colorado, Northern Arizona.
The generic name is derived from Tonto, an Indian name applied to the basal sandstone of the Cambrian series in the Grand Canyon region.
TONTOIA KWAGUNTENSIS, new species
Plate 24, fig. 4
This unusual form has a convex dorsal shield divided into a cephalon, thorax, and pygidium. The cephalon has a narrow, raised margin with the lateral third on each side of the cephalon rising rapidly to the elevated central section. The central section is slightly flattened and has a sharp median ridge extending from the anterior end back to the posterior margin. A similar ridge crosses each thoracic segment and extends back on the pygidium about one-fourth its length.
The segmentation of the cephalon is suggested by two narrow ridges crossing the right lateral space beneath the elevated central portion.
There is a suggestion of the presence of eyes on the posterior outer edge of the elevated central portion of the cephalon.
The thorax is divided into four segments by rounded, elevated, narrow ridges as shown by figure 4.
The pygidium is shorter and smaller than the cephalon, but I think this may be owing to the breaking away of the margin.
There is only one specimen and that is a matrix in a fine, hard sandstone. Trails that appear to have been made by this or a similar form occur on the surface of several of the layers of sandstone adjoining the one on which the specimen illustrated occurred.
I am not at all sure that this species should be placed with the trilobites, but with such forms as those illustrated by figures 3 and 5 at the same geological horizon it seems best to classify it with them for the present.
The total length of the specimen illustrated is 25 mm. Other proportions are shown by figure 4.
Formation and locality.—Middle Cambrian: (73) Tonto sandstone, upper portion, Kwagunt Valley, Grand Canyon of the Colorado, Northern Arizona.
Sub-Class MEROSTOMATA
Order AGLASPINA, new order
Body elongate, transversely trilobed. Cephalo-thorax with or without sessile eyes; on the ventral side it has an epistoma and five pairs of movable appendages.
Thorax with 8 to 11 segments, each of which has a pair of jointed appendages. Abdomen with 1 to 3 segments.
One family, Aglaspidæ Clarke.
Family AGLASPIDÆ Clarke
MOLARIA, new genus
The description of Molaria is outlined in that of the type species.
Genotype.—Molaria spinifera, new species.
Stratigraphic range.—The stratigraphic range is limited to a thin layer of dark siliceous shale about 2 inches in thickness forming the base of the phyllopod bed of the Burgess shale member of the Stephen formation.
Geographic distribution.—On the slope of the ridge between Wapta Peak and Mount Field, north of Burgess Pass, and about 3800 feet above Field on the line of the Canadian Pacific Railway, British Columbia, Canada.
The generic name is derived from Molar, the name of one of the mountain peaks east of the "Valley of the Ten Peaks," south of Laggan, Alberta, Canada.
The family reference is tentative.
MOLARIA SPINIFERA, new species
Plate 29, figs. 1-5
The dorsal test, when flattened on the shale and viewed from above, is elongate-oval in outline with a long, slender telson; it is obscurely trilobed longitudinally. Surface slightly roughened by minute shallow punctæ. Cephalic shield semicircular in outline, moderately convex. It is divided into a central area with a conical outline corresponding to the glabella of the trilobite and three transverse lobes are indicated by short furrows on each side. Postero-lateral angles without genal spines.
The examination of over 20 specimens has failed to reveal any traces of an eye on the cephalic shield. This might escape observation, but from the close relation of this species to the species in the genus Habelia it is probable that the eyes were pedunculated and beneath the rim of the cephalic shield.
Thorax with eight transverse segments divided into a median and two lateral lobes. The form and arrangement of the segments is clearly shown by figure 2. Abdomen with one long segment and a slender, spine-like telson.
Appendages.—The appendages of the head are not satisfactorily preserved. In one (fig. 5) a pair of short, jointed antennules (a') may be traced by their impression on the test to where they extend beyond the rim of the cephalic shield. On the left a larger and longer appendage may possibly represent an antenna. Several specimens show slender jointed appendages projecting from beneath the edge of the cephalic shield; these terminate in a minute joint having several fine setæ or spines on its margin.
On the thoracic leg two or three of the inner joints are widened out and setiferous; the outer three joints are long and slender; on the outer side of the inner joints (exopodite probably) there is a broad flabellum-like setiferous lobe, also a small oval gill (fig. 3). From beneath the posterior margin of the long abdominal segment shown in figure 4, a minute jointed leg projects on each side of the base of the telson.
Interior structure.—The alimentary canal is outlined in several specimens. On one of them (fig. 2) it extends to the posterior end of the long abdominal segment, thus indicating the position of the anal opening. Traces of the hepatic cæca beneath the head are shown by figure 3.
Dimensions.—This is a small species. The largest specimen has a length, exclusive of the telson, of 40 mm. The average length is about 18 to 20 mm. The proportions of the dorsal shield and telson are indicated by figure 2.
Observations.—I have employed above the terminology used in describing the dorsal shield of trilobites as it applies so well to this interesting species. In outward aspect the dorsal shield is essentially that of a trilobite except that there are no sessile eyes on the head shield and the posterior segments are more nearly related in form to such branchiopod crustaceans as Emeraldella brocki (pl. 30, fig. 2).
So far as can be determined, a pair of jointed legs, with an exopodite bearing a gill and flabellum, occurs on the outer side of each of the eight thoracic segments. A moderate number of specimens of the species were found in 1910 and it may be that more will be collected during the season of 1912 and that among them the cephalic appendages will be preserved. The test is thin and easily wrinkled, which obscures parts that would otherwise show through it.
Formation and locality.—Middle Cambrian, (35k) Burgess shale member of the Stephen formation, on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.
HABELIA, new genus
The description of the genus is included with that of the type species.
Genotype.—Habelia optata, new species.
Stratigraphic range.—The stratigraphic range is limited to a layer in a band of dark siliceous shale about 4 feet in thickness, forming a part of the Burgess shale member of the Stephen formation.
Geographic distribution.—On the slope of the ridge between Wapta Peak and Mount Field, north of Burgess Pass, and about 3800 feet above Field on the line of the Canadian Pacific Railway, British Columbia, Canada.
Observations.—The generic name is derived from the name Habel as applied to a mountain peak near Wapta glacier at the head of Yoho Valley, British Columbia, Canada.
HABELIA OPTATA, new species
Plate 29, fig. 6
In form the body of this species resembles Yohoia tenuis, but its long slender telson and thoracic appendages quickly separate it.
The test of the head is finely punctate, and crenulated with minute short spines on the margin. There are eleven body segments bearing appendages that are of nearly uniform size, one smaller abdominal segment without appendages, and the slender telson.
The eye has not been observed in any of the four specimens preserving the head.
Appendages.—On one specimen six slender jointed appendages project forward from beneath the carapace; the posterior of these is a little larger than the others. They represent, in front at least, the first five pairs of appendages of the head. Another specimen shows two slender jointed antennules and posterior to (below) them two larger jointed antennæ with delicate spines extending forward; another antenna is indicated by a small jointed leg-like appendage. The thoracic appendages are somewhat difficult to interpret. One specimen shows the broad terminal joint on eight legs, with traces of it on the three anterior legs; another specimen has five long, slender, jointed legs with a sixth posterior to them that is not over one-half as long; back of the latter there are five of the short legs with the broad terminal joint. The five anterior legs have a rather large gill attached to a short exopodite (?).
The terminal joints of the posterior legs have almost the outline of the pleuron of the segments of the thorax; they gently curve from a broad base to a fine point and have five or more short sharp spines on the anterior margin and somewhat finer spines on the posterior margin; their function was probably natatory.
Surface.—The surface of the head test and tergite of the segments is finely punctate. The telson appears to have been longitudinally striate or smooth.
Dimensions.—The largest specimen has a length of 22 mm. exclusive of the long telson, which is about as long as the body.
Observations.—The presence of this species in the collection was not noted until too late to illustrate it thoroughly. This will now be left until a further study can be made of it.
Formation and locality.—Middle Cambrian: (35k) Burgess shale member of the Stephen formation, on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.
EMERALDELLA, new genus
The description of this genus is outlined in the description of the type species.
Genotype.—Emeraldella brocki, new species.
Stratigraphic range.—The stratigraphic range is limited to a band of dark siliceous shale about 4 feet in thickness forming a part of the Burgess shale member of the Stephen formation.
Geographic distribution.—On the slope of the ridge between Wapta Peak and Mount Field, north of Burgess Pass, and about 3800 feet above Field on the line of the Canadian Pacific Railway, British Columbia, Canada.
Observations.—This genus appears to come within the limits of the family Aglaspidæ.
The generic name is derived from the name Emerald as applied to a mountain, lake, and glacier north of Burgess Pass, British Columbia, Canada.
EMERALDELLA BROCKI, new species
Text figure 8, p. 204, and plate 30, fig. 2
Body elongate, strong. As flattened in the shale it is about twice as long as its greatest diameter. Cephalon transversely semicircular. Eyes unknown. Thorax with ten segments bearing appendages. Abdomen with two long anterior segments and a short segment to which is joined a long, slender spine-like telson. Epistoma elongate and a little more than one-half the length of the cephalon.
Appendages.—The base of an antenna is fairly well shown by a specimen in which the head has been largely broken away (a', fig. 2, pl. 30). It has a thick, jointed basal portion. The two slender, jointed appendages projecting below the head on figure 2 may be the outward extensions of the maxillula (mx') and maxilla (mx").
A specimen found in 1911 shows a short antennule (text figure 8), very long slender antennæ, and three cephalic appendages.
The thoracic limbs are not well preserved, but what is shown indicates a broad, large terminal joint somewhat similar to that of Opabinia regalis (pl. 27, fig. 6) and another inner joint that is expanded and provided about its margin with strong setæ. The gill appears to be present but it cannot be satisfactorily determined.
Alimentary canal.—This canal is large and may be traced from the last abdominal segment through and into the head as shown in figure 9.
| Dimensions. | mm. |
| Length of body | 40 |
| Caudal spine | 30 |
| Head | 9 |
| Thorax | 26 |
| Abdomen | 4 |
 Fig. 8.—Emeraldella brocki, n. sp. Dorsal view of a flattened specimen. ✕ 1.5, showing antennules, antennæ, 3 posterior cephalic appendages, epistoma, and position of mouth. |
Observations.—The specific name is given in honor of R. W. Brock, Director of the Geological Survey of Canada, who has been most courteous to me in connection with my work in the Canadian Rocky Mountains.
Formation and locality.—Middle Cambrian: (35) Burgess shale member of the Stephen formation, on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.

Fig. 9.—Emeraldella micrura, n. sp. Type specimen
Natural size.
EMERALDELLA MICRURA, new species
Text figure 9
This species is founded on a single weathered specimen that we found in the shale about 80 feet above Emeraldella brocki. It differs from the latter mainly in its greater proportional width.
Formation and locality—Middle Cambrian: Burgess shale member of the Stephen formation (about 75 feet above the phyllopod bed near the base of the shale) on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.
Order LIMULAVA Walcott
- Limulava Walcott, 1911, Smithsonian Misc. Coll., Vol. 57, No. 2, p. 21. (Described as a new sub-order.)
Family SIDNEYIDÆ Walcott
Genus SIDNEYIA Walcott
SIDNEYIA INEXPECTANS Walcott
Text figure 10, p. 206
- Sidneyia inexpectans Walcott, 1911, Smithsonian Misc. Coll., Vol. 57, No. 2, p. 24, pl. 2, figs. 1-3, pl. 3, figs. 1-4, pl. 4, figs. 1-4, pl. 5, figs. 1-3, pl. 6, fig. 3, and pl. 7, fig. 1. (Original description and illustrations.)
A specimen found during the summer of 1911, text figure 10, shows the form of the head and four pairs of the cephalic appendages projecting forward in fine shape, but unfortunately the chelate terminal joints of the third pair are not attached. The antennæ are not preserved. The biramous appendages of the first 9 segments of the body are formed of an endopodite of four or five broad, short joints and two slender distal joints. Each joint has one or more short, sharp spines curving forward from the outer end, and the terminal joint has two large and one small forward-curving spines not unlike the spines on the terminal joints of the cephalic appendages of Eurypterus. In other specimens the exopodite appears to be in the form of a lobe or lamellæ not unlike the branchial lobes of Pterogotus bilobus as illustrated by Dr. Henry Woodward.[52] The branchial appendages of Sidneyia are illustrated in my paper on Middle Cambrian Merostomata.[53]
 Fig. 10.—Sidneyia inexpectans Walcott. ✕ 3. Flattened dorsal shield with 4 pairs of cephalic appendages, and jointed setiferous thoracic legs. |
The discovery of the jointed body legs of Sidneyia strengthens the conclusion reached in 1911, that Sidneyia was a transition form between the Trilobita and Eurypterida.
The accompanying text figure illustrates one of the specimens of Sidneyia showing the jointed body appendages.
For description of mode of occurrence and locality, see original description.
| DESCRIPTION OF PLATE 24 | ||||
| PAGE | ||||
| Neolenus serratus (Rominger) | 190 | |||
| Fig. | 1. | (Natural size.) A partly exfoliated specimen, showing (a') an antenna, numerous thoracic legs (thl), and jointed caudal rami (cr). The caudal rami have been dragged backward, pulling with them a portion of the under edge of the ventral lining of the body cavity. U. S. National Museum, Catalogue No. 57656. | ||
| 1a. | (Natural size.) Pygidium with the caudal rami extending out from beneath it in their probable natural position. U. S. National Museum, Catalogue No. 57657. | |||
| Ptychoparia cordilleræ (Rominger) | 190 | |||
| Fig. | 2. | (✕6.) Dorsal view of a specimen from which the dorsal shield has been removed, leaving the branchiæ (br) exposed; also a few of the thoracic legs (thl). U. S. National Museum, Catalogue No. 57658. | ||
| Mollisonia symmetrica Walcott | 196 | |||
| Fig. | 3. | (Natural size.) Dorsal view of the type specimen found in the Stephen formation on Mount Stephen above Field, locality (14s). U. S. National Museum, Catalogue No. 57659. | ||
| Tontoia kwaguntensis Walcott | 199 | |||
| Fig. | 4. | (✕2.) Dorsal view of a cast made in the natural mold, which is the type specimen. From the Tonto sandstone, Kwagunt Valley, Grand Canyon, Arizona, locality (73). U. S. National Museum, Catalogue No. 57660. | ||
| Mollisonia gracilis Walcott | 197 | |||
| Fig. | 5. | (✕2.5.) Dorsal view of the type specimen of the species and genus. The specimen is laterally compressed. U. S. National Museum, Catalogue No. 57661. | ||
| Mollisonia ? rara Walcott | 198 | |||
| Fig. | 6. | (Natural size.) A fragment of this species, showing the form of the ends of the thoracic segments. U. S. National Museum, Catalogue No. 57662. | ||
| 7. | (✕2.) A small crushed specimen, showing a portion of the cephalic shield, thoracic segments, and pygidium. U. S. National Museum, Catalogue No. 57663. | |||
The specimens represented by figures 1, 1a, 2, 5, 6, and 7 are from locality (35k) Middle Cambrian: Burgess shale member of the Stephen formation, on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.

DESCRIPTION OF PLATE 25
| Legend: | |
| c = carapace. | m=third appendage=mandible. |
| x=posterior spines or lobes of carapace. | m'=fourth appendage=maxillula. |
| e=eye. | m"=fifth appendage=maxilla. |
| lb=labrum. | i=intestine or alimentary canal. |
| th=thorax. | thi=thoracic legs [endopodites]. |
| ab=abdomen. | l=setiferous jointed exopodite. |
| a' = antennule ?. | br=branchial lobes or gills [epipodites]. |
| a"=antennæ. |
| PAGE | ||||
| Marrella splendens Walcott (see pl. 26) | 193 | |||
| Fig. | 1. | (✕4.) Dorsal view of a crushed specimen in which the juncture of the antennules ? (a') and the carapace is clearly shown. U. S. National Museum, Catalogue No. 57664. | ||
| 2. | (✕4.) Dorsal view illustrating the segments of the body, carapace and its great posterior spines. U. S. National Museum, Catalogue No. 57665. | |||
| 3. | (✕4.) Dorsal view of a partly exfoliated specimen showing the antennæ (a"), a feather-like maxillula, and one of the remarkable setiferous exopodites. U. S. National Museum, Catalogue No. 57666. | |||
| 4. | (✕3.) A specimen crushed sideways so as to force the eye (e) out prominently on the left side. U. S. National Museum, Catalogue No. 57667. | |||
| 5. | (✕3.) A sliglitly distorted specimen illustrated to show the eyes (e). U. S. National Museum, Catalogue No. 57668. | |||
| 6. | (✕4.) Dorsal view of a specimen showing the setiferous exopodites as they lie one on the other with the setæ pointing forward and outward. U. S. National Museum, Catalogue No. 57669. | |||
All of the specimens illustrated on Plate 25 are from locality (35k) Middle Cambrian: Burgess shale member of the Stephen formation, on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.

DESCRIPTION OF PLATE 26
| Legend: | |
| c = carapace. | m=third appendage=mandible. |
| x=posterior spines or lobes of carapace. | m'=fourth appendage=maxillula. |
| e=eye. | m"=fifth appendage=maxilla. |
| lb=labrum. | i=intestine or alimentary canal. |
| th=thorax. | thi=thoracic legs [endopodites]. |
| ab=abdomen. | l=setiferous jointed exopodite. |
| a'=antennule ?. | br=branchial lobes or gills [epipodites]. |
| a"=antennæ. |
| PAGE | ||||
| Marrella splendens Walcott (see pl. 25) | 193 | |||
| Fig. | 1. | (✕3.) Dorsal view of a specimen showing the carapace with its great posterior spines or lobes and the large antennules?. U. S. National Museum, Catalogue No. 57670. | ||
| 2. | (✕3.) Ventral view of a crushed specimen showing the labrum (hypostoma) nearly in its normal position. U. S. National Museum, Catalogue No. 57671. | |||
| 3. | (✕3.) Dorsal view of a specimen showing intestinal canal, feather-like maxillula (m), and the long joints of the setiferous exopodites. U. S. National Museum, Catalogue No. 57672. | |||
| 4. | (✕4.) Ventral view of a specimen showing the large gill lobes (br) (epipodites). U. S. National Museum, Catalogue No. 57673. | |||
| 5. | (✕3.) Dorsal view of a specimen that illustrates the relative position of the antennæ, the mandible, maxillula, and maxilla. U. S. National Museum, Catalogue No. 57674. | |||
| 6. | (✕4.) Ventral view of a specimen showing the thoracic legs (thl, endopodites) and the setiferous legs (l, exopodite). U. S. National Museum, Catalogue No. 57675. | |||
All of the specimens illustrated on Plate 26 are from locality (35k) Middle Cambrian: Burgess shale member of the Stephen formation, on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.

| DESCRIPTION OF PLATE 27 | ||||
| PAGE | ||||
| Burgessia bella Walcott (see pl. 30) | 177 | |||
| Fig. | 1. | (✕3.) Dorsal view of a specimen showing the internal structure through the thin carapace. st = stomach, i = intestinal canal, kd = hepatic cæca, cl = connection between hepatic cæca and alimentary canal, thl = thoracic legs, ab = abdominal region, but the segmentation is not preserved in this specimen. U. S. National Museum, Catalogue No. 57676. | ||
| 2. | (✕3.) Ventral view of the region beneath the carapace. Lettering the same as for figure 1 with the addition of a' = antennæ, lb = labrum. U. S. National Museum, Catalogue No. 57677. | |||
| 3. | (✕3.) Dorsal view of area within the cephalic shield, showing interior and appendages of the head. e = eye, a" = antennæ, 3, 4, 5 = cephalic appendages, th = base of thoracic appendage. Other lettering as in figures 1 and 2. U. S. National Museum, Catalogue No. 57678. | |||
| Waptia fieldensis Walcott | 181 | |||
| Fig. | 4. | (✕1.5.) Dorsal view of a specimen flattened on the shale. c = carapace, e = eye, a' = antennæ, thl = thoracic legs, cr = caudal rami. U. S. National Museum, Catalogue No. 57681. | ||
| 5. | (✕2.) Side view with carapace (c) pushed over forward so as nearly to reverse the position of the dorsal line. th = thoracic segments, thl = thoracic legs, ab = abdominal segments, cr = caudal rami, m = mandible, m' = maxillula, m" = maxilla, br = gill lobe. U. S. National Museum, Catalogue No. 57682. | |||
| Opabinia regalis Walcott (see pl. 28, fig. 1) | 167 | |||
| Fig. | 6. | (✕2.) Lateral view of the type specimen of the male of the species. The eyes (e) are crushed one beside the other. fp = frontal appendage, ths = trunk somites, br = gills, f = terminal lobe-like joints of thoracic legs, t = telson or terminal somite. U. S. National Museum, Catalogue No. 57683. | ||
All of the specimens illustrated on Plate 27 are from locality (35k) Middle Cambrian: Burgess shale member of the Stephen formation, on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.

| DESCRIPTION OF PLATE 28 | ||||
| PAGE | ||||
| Opabinia regalis Walcott (see pl. 27, fig. 6) | 167 | |||
| Fig. | 1. | (✕2.) Dorsal view of a male specimen, flattened in the shale, showing fp = frontal appendage, e = eye, ths = thoracic somites, i = intestine, ab = abdominal segment. U. S. National Museum, Catalogue No. 57684. | ||
| Nathorstia transitans Walcott | 194 | |||
| Fig. | 2. | (✕2.) Dorsal view of the type specimen of the species. It is flattened and slightly distorted with the left side more or less crushed in towards the center. a' = antenna, e = eye, gs = genal spine, pl = pleura of thoracic segments, m = 4 joints of leg (mandible?), ta = central or thoracic axis, i = intestine, thl = thoracic legs, cr = caudal rami. U. S. National Museum, Catalogue No. 57685. | ||
| Naraoia compacta Walcott | 175 | |||
| Fig. | 3. | (✕2.) Side view of cephalo-thoracic carapace with traces of appendages showing through it. U. S. National Museum, Catalogue No. 57686. | ||
| 4. | (✕2.) Dorsal view of specimen described. U. S. National Museum, Catalogue No. 57687. | |||
All of the specimens illustrated on Plate 28 are from locality (35k) Middle Cambrian: Burgess shale member of the Stephen formation, on the west slope of the ridge betwen Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.

| DESCRIPTION OF PLATE 29 | ||||
| PAGE | ||||
| Molaria spinifera Walcott | 200 | |||
| Fig. | 1. | (✕3.) Side view in which the thoracic pleuræ of the segments have been broken away so as to show appendages. U. S. National Museum, Catalogue No. 57688. | ||
| 2. | (✕3.) Dorsal view. U. S. National Museum, Catalogue No. 57689. | |||
| 3. | (✕3.) Ventral view, showing some of the gills (br), liver (k) beneath the cephalic shield, and alimentary (al) canal. The ends of the thoracic segments (ths) are marked by fine lines. U. S. National Museum, Catalogue No. 57690. | |||
| 4. | (✕3.) Dorsal view. Minute appendages show from beneath abdominal segment, also setiferous joints of the thoracic legs. U. S. National Museum, Catalogue No. 57691. | |||
| 5. | (✕3) Dorsal view. a' = antennules. U. S. National Museum, Catalogue No. 57692. | |||
| Habelia optata Walcott | 202 | |||
| Fig. | 6. | (✕2.) Side view of a specimen flattened in the shale. Three of the setiferous legs near the head have been pushed out above the body. U. S. National Museum, Catalogue No. 57693. | ||
| Yohoia tenuis Walcott | 172 | |||
| Fig. | 7. | (✕2.) Side view showing the pleuræ of the thorax and traces of the appendages of the head. U. S. National Museum, Catalogue No. 57694. | ||
| 8. | (✕3.) Specimen with abdomen and expanded rami forming a swimming tail. U. S. Museum, Catalogue No. 57695. | |||
| 9. | (✕2.) Fragment of thorax and head, showing segmentation of thorax, eyes, antennule in center and part of a large antenna. The latter has its spiniferous terminal joint turned down towards the eye on the right side (upper in drawing). U. S. National Museum, Catalogue No. 57696. | |||
| 10. | and 11. (✕2.) Small specimens seen from above. U. S. National Museum, Catalogue Nos. 57697 and 57698. | |||
| 12. | (✕2.) Side view of a specimen showing segmentation of head and thorax; antenna and portions of thoracic appendages. U. S. National Museum, Catalogue No. 57699. | |||
| 13. | (✕3.) The anterior half of figure 12, still further enlarged. | |||
| Yohoia plena Walcott | 173 | |||
| Fig. | 14. | (✕3.) A small specimen showing the general form of the species. Better specimens have been found since the plate was prepared and will be illustrated in a future paper. U. S. National Museum, Catalogue No. 57700. | ||
All of the specimens illustrated on Plate 29 are from locality (35k) Middle Cambrian: Burgess shale member of the Stephen formation, on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.
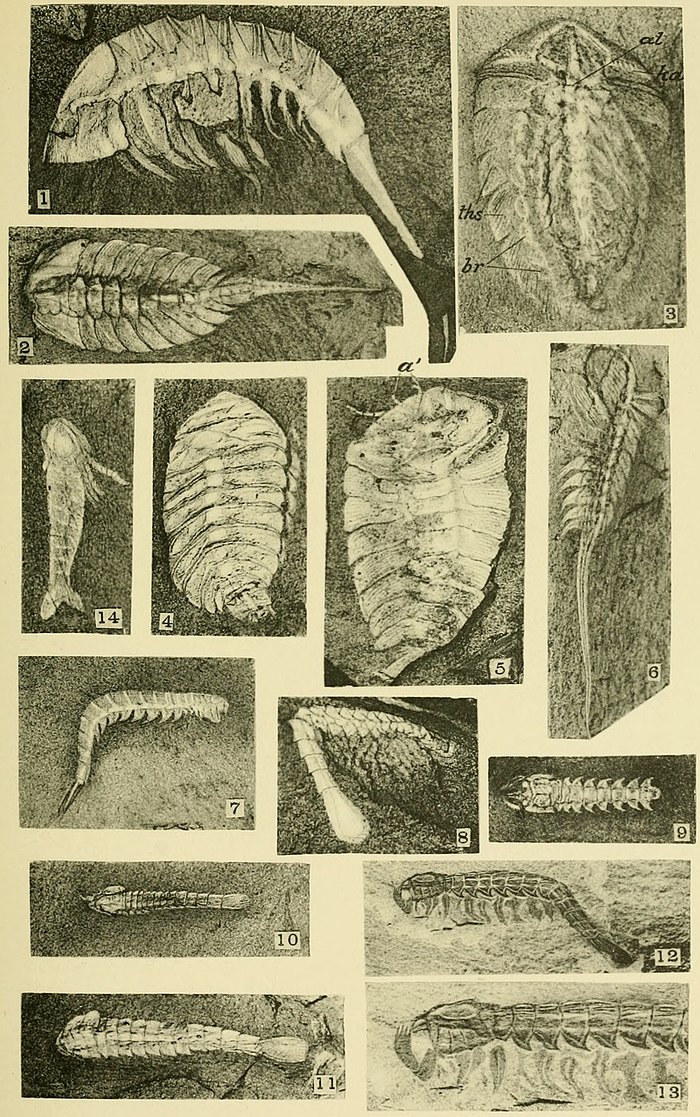
| DESCRIPTION OF PLATE 30 | ||||
| PAGE | ||||
| Bidentia difficilis Walcott | 174 | |||
| Fig. | 1. | (✕2.) Side view of a flattened specimen that has been injured by water percolating through the shale. The antennæ (a') are fairly well shown. U. S. National Museum, Catalogue No. 57701. | ||
| Emeraldella brocki Walcott | 203 | |||
| Fig. | 2. | (✕2.) A specimen flattened out so as to give a partial view of the head and body and a fine profile view of the abdomen and telson. e = eye, a' = antenna, mx' = maxillula, mx" = maxilla, thl = thoracic legs, i = alimentary canal. U. S. National Museum, Catalogue No. 57702. | ||
| Burgessia bella Walcott (see pl. 27) | 177 | |||
| Fig. | 3. | (✕3.) Profile view of a specimen with carapace crushed and distorted. U. S. National Museum, Catalogue No. 57679. | ||
| 4. | (✕3.) Side view of another specimen in which the carapace has been crowded off the body and out of shape. U. S. National Museum, Catalogue No. 57680. | |||
The specimens illustrated on Plate 30 are from locality (35k) Middle Cambrian: Burgess shale member of the Stephen formation, on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.

| DESCRIPTION OF PLATE 31 | ||||
| PAGE | ||||
| Hymenocaris perfecta Walcott | 183 | |||
| Fig. | 1. | (✕2.) Side view of the right valve, showing the form of the valve, abdomen, and numerous appendages. Adductor muscle scar (ad), intestine (i), thoracic legs (thl), gills (br). U. S. National Museum, Catalogue No. 57703. | ||
| 2. | (✕1.5.) Dorsal view of a specimen from which the test has been exfoliated. This shows the antenna (a), adductor muscle scars (ad), intestine (i), and traces of the basal joints of the legs. U. S. National Museum, Catalogue No. 57704. | |||
| 3, | 4, and 5. (✕2.) Illustrations of the posterior end of the abdomen with cercopods. U. S. National Museum, Catalogue Nos. 57705, 57706, and 57707. | |||
| 6. | Specimen in upper left hand corner of figure. (Natural size.) Dorsal view of a crushed speicmen showing the carapace, abdomen, and cercopods. U. S. National Museum, Catlogue No. 57708. | |||
| Leanchoilia superlata Walcott | 170 | |||
| Fig. | 6. | Lower specimen. (Natural size.) Side view of the type and only specimen known of the species and genus. U. S. National Museum, Catalogue No. 57709. | ||
All of the specimens illustrated on Plate 31 are from locality (35k) Middle Cambrian: Burgess shale member of the Stephen formation, on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.

| DESCRIPTION OF PLATE 32 | ||||
| PAGE | ||||
| Hymenocaris obliqua Walcott | 185 | |||
| Fig. | 1. | (Natural size.) Side view of the type specimen of the species, showing carapace, abdomen, and cercopods. U. S. National Museum, Catalogue No. 57710. | ||
| 2. | (Natural size.) Side view of the left valve of the carapace compressed so as to shorten it. U. S. National Museum, Catalogue No. 57711. | |||
| 3. | (Natural size.) Side view of a right valve that has been compressed so as to shorten it a little. U. S. National Museum, Catalogue No. 57712. | |||
| Hymenocaris ? circularis Walcott | 184 | |||
| Fig. | 4. | (✕3.) A somewhat distorted valve with a number of legs projecting below its margin. U. S. National Museum, Catalogue No. 57713. | ||
| Hymenocaris ovalis Walcott | 185 | |||
| Fig. | 5. | (✕3.) Side view of the right valve showing the general form. U. S. National Museum, Catalogue No. 57714. | ||
| 6. | (✕3.) Dorsal view of a carapace, abdomen, and two cercopods. U. S. National Museum, Catalogue No. 57715. | |||
| Hymenocaris ? parva Walcott | 185 | |||
| Fig. | 7. | (✕4.) Type specimen of the species and genus, showing several appendages and the abdomen turned forward on the carapace. U. S. National Museum, Catalogue No. 57716. | ||
| Fieldia lanceolata Walcott | 188 | |||
| Fig. | 8. | (✕2.) Side view, left valve, of the type specimen, illustrating characters described. U. S. National Museum, Catalogue No. 57717. | ||
| Hurdia victoria Walcott | 186 | |||
| Fig. | 9. | (Natural size.) Side view of the left valve. U. S. National Museum, Catalogue No. 57718. | ||
All of the specimens illustrated on Plate 32 are from locality (35k) Middle Cambrian: Burgess shale member of the Stephen formation, on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.

| DESCRIPTION OF PLATE 33 | ||||
| PAGE | ||||
| Carnarvonia venosa Walcott | 189 | |||
| Fig. | 1. | (Natural size.) Dorsal view of the two valves of the carapace flattened on the surface of the shale. The adductor muscle scar (adm) shows through the test, and the fine venation extending from the head portion backward is beautifully shown. U. S. National Museum, Catalogue No. 57719. | ||
The specimen illustrated by fig. 1 is from locality Middle Cambrian: Burgess shale member of the Stephen formation (about 75 feet above the phyllopod bed near the base of the shale), on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.
| Tuzoia retifera Walcott | 187 | |||
| Fig. | 2. | (Natural size.) View of flattened right valve showing reticulate surface markings. U. S. National Museum, Catalogue No. 57720. | ||
The specimen illustrated by fig. 2 is from locality (35k) Middle Cambrian: Burgess shale member of the Stephen formation, on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.

| DESCRIPTION OF PLATE 34 | ||||
| PAGE | ||||
| Hurdia triangulata Walcott | 186 | |||
| Fig. | 1. | (Natural size.) Typical form of valve (right). U. S. National Museum, Catalogue No. 57721. | ||
| Odaraia alata Walcott | 188 | |||
| Fig. | 2. | (Natural size.) A right valve with the end of the abdomen and two cercopods projecting from posterior end. The latter are evidently displaced. U. S. National Museum, Catalogue No. 57722. | ||
| Anomalocaris gigantea Walcott | 180 | |||
| Fig. | 3. | (Natural size.) Portion of the abdomen showing segments and appendages. U. S. National Museum, Catalogue No. 57723. | ||
All of the specimens illustrated on Plate 34 are from locality (35k) Middle Cambrian: Burgess shale member of the Stephen formation, on the west slope of the ridge between Mount Field and Wapta Peak, one mile (1.6 km.) northeast of Burgess Pass, above Field, British Columbia.

INDEX
Note.—The first reference to each of the species described gives the page upon which the description begins and the figure references. References to the description of certain parts or features of a species are as a rule only given in the index if the description occurs outside of the detailed description of the species. For instance: the description of the pygidium of a certain species will be found in the description of that species and there will be no specific reference in the index to the pygidium unless it is described or discussed at some other point in the paper.
The references in heavy-faced type refer to the pages upon which the species are described.
| PAGE | |||
| Acidaspis, compared with Marrella (footnote) | 162 | ||
| Aglaspidæ Clarke | 200 | ||
| classification of | 155 | ||
| Emeraldella referred to | 203 | ||
| possible relation of trilobite to | 163 | ||
| Aglaspina, new order, described | 199 | ||
| classification of | 155 | ||
| derived from Trilobita | 163 | ||
| foreshadowed by Burgess shale fauna | 165 | ||
| relation to Limulava | 164 | ||
| Aglaspis, representing Merostomata | 155 | ||
| Agnostus, compared with Mollisonia | 196 | ||
| mentioned | 149, 151 | ||
| alata, see Odaraia. | |||
| Algonkian, genera that occur in | 155 | ||
| Alimentary canal, species preserving | 160 | ||
| Amiella, characterized | 158 | ||
| representing Merostomata | 155 | ||
| mentioned | 148 | ||
| Amiskwia sagittiformis, stratigraphic position and association | 152 | ||
| Ampullaria, ancestors of | 166 | ||
| Analysis, chemical, of slate | 150 | ||
| Anomalocaris, abdominal segments of, | 159 | ||
| Whiteaves | 154, 180 | ||
| Anomalocaris canadensis Whiteaves, associated with Mollisonia symmetrica | 197 | ||
| compared with Anomalocaris gigantea | 180 | ||
| Anomalocaris gigantea, new species | 180, pl. 34, fig. 3 | ||
| compared with Anomalocaris canadensis Whiteaves | 180 | ||
| stratigraphic position and association | 153 | ||
| Anostraca Calman | 166 | ||
| classification of | 154 | ||
| PAGE | |||
| Anostraca, foreshadowed by Burgess shale fauna | 165 | ||
| in Cambrian time | 165 | ||
| no true shell among | 157 | ||
| recent species of, compared with Opabinia | 169 | ||
| telson | 157 | ||
| Antennæ, Branchiopoda and Malacostraca | 159 | ||
| Antennules, Branchiopoda and Malacostraca | 159 | ||
| Apodidæ, Apus and other genera of | 162 | ||
| compared with Burgessia bella | 179, 180 | ||
| preservation of | 166 | ||
| remarkable adaptation of | 165 | ||
| theoretical ancestral stock correlated with | 163 | ||
| Appendages, described and discussed | 158-160 | ||
| of Neolenus and Ptychoparia | 190 | ||
| Apus, Bernard's study of | 162 | ||
| compared with Marrella splendens | 192 | ||
| parapodia of | 162 | ||
| posterior segments of | 159 | ||
| separated from Burgessia | 162-163 | ||
| mentioned | 191 | ||
| Asaphidæ, mentioned | 196 | ||
| Asaphus platycephalus, mentioned | 190 | ||
| Asheaia pedunculata, stratigraphic position and association | 153 | ||
| Asterocheridæ, hepatic cæca of | 160 | ||
| Banffia grandis, stratigraphic position and association | 153 | ||
| Bather, F. A., correction referring to | 148 | ||
| Bathyuriscus rotundatus (Rominger), associated with Mollisonia symmetrica | 197 | ||
| becki, see Triarthrus. | |||
| Beecher, Order Hypoparia | 195 | ||
| bella, see Burgessia. | |||
| Beltina, link between Merostomata and Ziphosuridæ | 165 | ||
| occurrence in pre-Cambrian | 162 | ||
| representing Merostomata | 155 | ||
| Bernard, "The Apodidæ" | 166 | ||
| "The Systematic Position of the Trilobites" quoted | 190-191 | ||
| study of Apus | 162 | ||
| Bident, mountain peak, Alberta, Canada | 174 | ||
| Bidentia, new genus, discussed | 173 | ||
| classification of | 154 | ||
| no living representatives | 165 | ||
| Bidentia difficilis, new species | 174, pl. 30, fig. 1 | ||
| appendages | 174 | ||
| compared with Emeraldella brocki | 174 | ||
| compared with Waptia fieldensis | 174 | ||
| stratigraphic position and association | 153 | ||
| Bohemilla stupenda Barrande, thorax of Mollisonia, recalls | 196 | ||
| PAGE | |||
| Branchinecta, specimens flattened between glass plates | 169 | ||
| Branchinecta paludosa (O. F. Müller), effect of pressing | 168 | ||
| Branchiopoda, classification of | 154 | ||
| Apodidæ of, compared with Burgessia bella | 179 | ||
| Bidentia difficilis nearer to Merostomata than to | 174 | ||
| Burgessia in | 163 | ||
| Cambrian, descendants of | 166 | ||
| descent of | 160-161 | ||
| development discussed | 163 | ||
| foreshadowed by Burgess shale fauna | 165 | ||
| genera in the Cambrian | 157 | ||
| of the order Anostraca | 165 | ||
| relations of, to Leptostraca, Malacostraca, Trilobita, and Merostomata | 163 | ||
| segmentation, table of | 158 | ||
| subclass | 148, 153, 166 | ||
| survival of | 165 | ||
| mentioned | 155 | ||
| Branchiopoda and Malacostraca, compared with Waptidæ | 180 | ||
| Branchipus, specimens flattened between glass plates | 169 | ||
| Branchipus vernalis Verrill, compared with Opabinia regalis | 168 | ||
| Brock, R. W., Director of Geological Survey, Canada, acknowledgments | 204 | ||
| brocki, see Emeraldella. | |||
| Burgess, mountain and pass, British Columbia, Canada | 177 | ||
| Burgess Pass, trail to Summit Lake from | 152 | ||
| Burgess shale, crustaceans of | 149 | ||
| Naraoia compacta from | 176 | ||
| species of ostracod in | 157 | ||
| Burgess shale fauna, foreshadows living Crustacea | 165 | ||
| relation to recent crustaceans | 164 | ||
| Burgess shale fossils, mentioned | 193 | ||
| Burgess shale member, collections from | 148 | ||
| of Stephen formation | 151 | ||
| Burgessia, new genus, discussed | 177 | ||
| classification of | 154 | ||
| carapace of | 157 | ||
| compared with Lepidurus | 165 | ||
| labrum of | 158 | ||
| probable ancestor of Polyartemidæ and Apodidæ | 165 | ||
| separated from Apus | 162-163 | ||
| shield of, mentioned | 157 | ||
| stratigraphic position and association | 153 | ||
| Burgessia bella, new species | 177, pl. 27, figs. 1-3, pl. 30, figs. 3 and 4 | ||
| appendages of | 178 | ||
| carapace of | 177-178 | ||
| compared with Apodidæ | 179 | ||
| compared with Apus | 162 | ||
| compared with Naraoia compacta | 176 | ||
| eyes of | 178 | ||
| stratigraphic position and association | 152 | ||
- ↑ Smithsonian Misc. Coll., Vol. 57, No. 2, 1911, pp. 17-40, pls. 2-7.
- ↑ Idem, No. 3, pp. 41-68, pls. 8-13, and No. 5, pp. 109-144, pls. 18-23.
- ↑ Idem, No. 3. pp. 43-45.
- ↑ What is an Echinoderm? Journal of the City of London College Science Society, Vol. 8, 1901, pp. 1-25.
- ↑ Smithsonian Misc. Coll., Vol. 57, No. 3, 1911, p. 42.
- ↑ Smithsonian Misc. Coll., Vol. 57, No. 3, 1911, p. 51.
- ↑ No attempt has been made to indicate the family relations of the large forms represented by the genera Hurdia, Tuzoia, and Odaraia. With our present knowledge of them they might possibly be referred to the Hymenocaridæ of Salter.
- ↑ Bull. Geol. Soc. America, Vol. 10, 1899, p. 238.
- ↑ Smithsonian Misc. Coll., Vol. 57, No. 3, 1911, p. 28.
- ↑ Idem, p. 27.
- ↑ Sixteenth Ann. Rept. New York State Museum, 1863, pp. 181 and 182, pl. II, figs. 7-16.
- ↑ American Journ. Sci., Vol. 12, 1901, pp. 364-366, pl. 7.
- ↑ Tenth Ann. Rept. U. S. Geol. Survey, 1891, pp. 625 and 626, pl. 80, figs. 10 and 10a.
- ↑ Tenth Ann. Rept. U. S. Geol. Survey, 1891, p. 626, pl. 80, figs. 1 and 1a. The genus Indiana Matthew is described in the Canadian Record of Science, Vol. 8, 1902, p. 460.
- ↑ Smithsonian Misc. Coll., Vol. 53, No. 6, 1911, pp. 249 and 258.
- ↑ Tenth Ann. Rept. U. S. Geol. Survey, 1891, p. 629, pl. 81, fig. 6.
- ↑ Challenger Rept., 1887, Vol. 19, Pt. 56, pl. 3, figs. 1, 5, and 6.
- ↑ 18.0 18.1 Smithsonian Misc. Coll., Vol. 57, 1910, pls. 2-7.
- ↑ Idem. pl. 5.
- ↑ Smithsonian Misc. Coll., Vol. 57, No. 1, 1910, p. 14.
- ↑ C. D. Walcott, 1899, Pre-Cambrian Fossiliferous Formations ; Bull. Geol. Soc. America, Vol. 10, pp. 238-239, pls. 25 and 26.
- ↑ The Apodidæ. Nature Series, London, 1892.
- ↑ Smithsonian Misc. Coll., Vol. 57, No. 5, 1911, pl. 23, fig. 4.
- ↑ Dr. Austin H. Clark considers that a comparison should be made between Marrella and the Trilobita. He suggests that the cephalon is comparable with that of Acidaspis, the two anterior spines being the genal spines and the posterior spines the same as the occipital spines or processes of Acidaspis. The terminal plate he takes to be the pygidium, and the feathery organ (m) the last cephalic appendage.
- ↑ Smithsonian Misc. Coll., Vol. 57, No. 2, 1911, p. 22.
- ↑ Smithsonian Misc. Coll., Vol. 57, No. 2, 1911, pp. 19-28, pls. 2-7.
- ↑ U. S. Geog. and Geol. Surv. Territories, 12th Ann. Rept., Pt. 1, 1883, pp. 353-355. Text-fig. 23.
- ↑ Challenger Rept. 1887, Vol. 19, Pt. 56, pl. 3, figs, 1, 5, and 6.
- ↑ Name proposed in MSS. by Ulrich and Bassler.
- ↑ This was referred to as a subfamily of the Eurypterida in 1911, but its characters are such that it now seems desirable to consider its typical genus, as representing a family of the Merostomata.
- ↑ The Apodidæ. Nature Series, London, 1892, p. 9.
- ↑ Walcott, Monogr. U. S. Geol. Survey, Vol. 8, 1884, p. 262.
- ↑ Idem, p. 261.
- ↑ As defined in Lankester's Treatise on Zoölogy, London, 1909, Pt. 7, p. 53.
- ↑ U. S. Geog. and Geol. Surv. Territories, 12th Ann. Rept., Pt. 1, 1883, pl. 14.
- ↑ U. S. Geog. and Geol. Surv. Territories, 12th Ann. Rept., Pt. 1, 1883, pp. 353-355, text fig. 23.
- ↑ As defined in Lankester's Treatise on Zoölogy, London, 1909, Pt. 7, p. 53.
- ↑ Canadian Alpine Journal, Vol. 1, No. 2, 1908, pl. 2, fig. 3a.
- ↑ Bull. U. S. Geol. Survey, No. 30, 1886, p. 146, pl. 8, fig. 5.
- ↑ Canadian Alpine Journ., Vol. 1, No. 2, 1908, pl. 2, fig. 5.
- ↑ Challenger Rept., Vol. 19, Pt. 56, 1887, pl. 3, figs. 1, 5, and 6.
- ↑ The Trilobite, New and Old Evidence relating to its Organization. Bull. Mus. Comp. Zoöl. at Harvard College, Vol. 8, 1881, pp. 208-211.
- ↑ The Organization of Trilobites. London, 1846, p. 46.
- ↑ The Systematic Position of the Trilobites. H. M. Bernard, Quart. Journ. Geol. Soc., London, Vol. 50, 1894, pp. 411-432; and Vol. 51, 1895, pp. 352-359.
- ↑ Burmeister, "The Organization of Trilobites," 1846, pp. 46-47.
- ↑ The Systematic Position of the Trilobites, 1894, Pt. 1, pp. 429-430.
- ↑ Idem, 1895, Pt. 2, p. 356.
- ↑ Smithsonian Misc. Coll., Vol. 57, No. 2, 1911. pl. 6, figs. 1 and 2.
- ↑ Smithsonian Misc. Coll., Vol. 57, No. 2, 1911, pl. 2, fig. 1; pl. 6, fig. 3; pl. 17, fig. 1.
- ↑ The Trilobite, New and Old Evidence relating to its Organization. Bull. Mus. Comp. Zoöl., Vol. 8, 1881, pp. 208-211.
- ↑ Systeme Silurien du Centre de la Bohême, Vol. I, Suppl, 1872, pl. 14, figs. 30 and 32.
- ↑ Monogr. British Fossil Crustacea, Order Merostomata, 1866-1878; Pal. Soc. London; Pl. 12, figs. 1a and 1d.
- ↑ Smithsonian Misc. Coll., Vol. 57, No. 2, 1911, pl. 6, fig. 3, and pl. 7, fig. 1.
![]()
This work is in the public domain in the United States because it was published before January 1, 1929.
The longest-living author of this work died in 1927, so this work is in the public domain in countries and areas where the copyright term is the author's life plus 96 years or less. This work may be in the public domain in countries and areas with longer native copyright terms that apply the rule of the shorter term to foreign works.
![]()
Public domainPublic domainfalsefalse
