CHAPTER XII.
Secondary Sexual Characters of Fishes, Amphibians, and Reptiles.
Fishes: Courtship and battles of the males—Larger size of the females—Males, bright colours and ornamental appendages; other strange characters—Colours and appendages acquired by the males during the breeding-season alone—Fishes with both sexes brilliantly coloured—Protective colours—The less conspicuous colours of the female cannot be accounted for on the principle of protection—Male fishes building nests, and taking charge of the ova and young. Amphibians: Differences in structure and colour between the sexes—Vocal organs. Reptiles: Chelonians—Crocodiles—Snakes, colours in some cases protective—Lizards, battles of—Ornamental appendages—Strange differences in structure between the sexes—Colours—Sexual differences almost as great as with birds.
We have now arrived at the great sub-kingdom of the Vertebrata, and will commence with the lowest class, that of Fishes. The males of Plagiostomous fishes (sharks, rays) and of Chimæroid fishes are provided with claspers which serve to retain the female, like the various structures possessed by many of the lower animals. Besides the claspers, the males of many rays have clusters of strong sharp spines on their heads, and several rows along "the upper outer surface of their pectoral fins." These are present in the males of some species, which have other parts of their bodies smooth. They are only temporarily developed during the breeding-season; and Dr. Günther suspects that they are brought into action as prehensile organs by the doubling inwards and downwards of the two sides of the body. It is a remarkable fact that the females and not the males of some species, as of Raia clavata, have their backs studded with large hook-formed spines.[1]
The males alone of the capelin (Mallotus villosus, one of Salmonidæ), are provided with a ridge of closely-set, brush-like scales, by the aid of which two males, one on each side, hold the female, whilst she runs with great swiftness on the sandy beach, and there deposits her spawn.[2] The widely distinct Monacanthus scopas presents a somewhat analogous structure. The male, as Dr. Günther informs me, has a cluster of stiff, straight spines, like those of a comb, on the sides of the tail; and these in a specimen six inches long were nearly one and a half inches in length; the female has in the same place a cluster of bristles, which may be compared with those of a tooth-brush. In another species, M. peronii, the male has a brush like that possessed by the female of the last species, whilst the sides of the tail in the female are smooth. In some other species of the same genus the tail can be perceived to be a little roughened in the male and perfectly smooth in the female; and lastly in others, both sexes have smooth sides.
The males of many fish fight for the possession of the females. Thus the male stickleback (Gasterosteus leiurus) has been described as "mad with delight," when the female comes out of her hiding-place and surveys the nest which he has made for her. "He darts round her in every direction, then to his accumulated materials for the nest, then back again in an instant; and as she does not advance he endeavours to push her with his snout, and then tries to pull her by the tail and side-spine to the nest."[3] The males are said to be polygamists;[4] they are extraordinarily bold and pugnacious, whilst "the females are quite pacific." Their battles are at times desperate; "for these puny combatants fasten tight on each other for several seconds, tumbling over and over again, until their strength appears completely exhausted." With the rough-tailed stickleback (G. trachurus) the males whilst fighting swim round and round each other, biting and endeavouring to pierce each other with their raised lateral spines. The same writer adds,[5] "the bite of these little furies is very severe. They also use their lateral spines with such fatal effect, that I have seen one during a battle absolutely rip his opponent quite open, so that he sank to the bottom and died." When a fish is conquered, "his gallant bearing forsakes him; his gay colours fade away; and he hides his disgrace among his peaceable companions, but is for some time the constant object of his conqueror's persecution."
The male salmon is as pugnacious as the little stickleback; and so is the male trout, as I hear from Dr. Günther. Mr. Shaw saw a violent contest between two male salmon which lasted the whole day; and Mr. R. Buist, Superintendent of Fisheries, informs me that he has often watched from the bridge at Perth the males driving away their rivals, whilst the females were spawning. The males "are constantly fighting and tearing each other on the spawning-beds, and many so injure each other as to cause the death of numbers, many being seen swimming near the banks of the river in a state of exhaustion, and apparently in a dying state."[6] Mr. Buist informs me, that in June 1868, the keeper of the Stormontfield breeding-ponds visited the northern Tyne and found about 300 dead salmon, all of which with one exception were males; and he was convinced that they had lost their lives by fighting.
The most curious point about the male salmon is that during the breeding-season, besides a slight change in colour, "the lower jaw elongates, and a cartilaginous projection turns upwards from the point, which, when the jaws are closed, occupies a deep cavity between the intermaxillary bones of the upper jaw."[7] (Figs. 27 and 28.) In our salmon this change of structure lasts only during the breeding-season; but in the Salmo lycaodon of N.-W. America the change, as Mr. J. K. Lord[8] believes, is permanent, and best marked in the older males which have previously ascended the rivers. In these old males the jaw becomes developed into an immense hook-like projection, and

Fig. 27. Head of male common salmon (Salmo salar) during the breeding-season.
[This drawing, as well as all the others in the present chapter, have been executed by the well-known artist, Mr. G. Ford, from specimens in the British Museum, under the kind superintendence of Dr. Günther.]
the teeth grow into regular fangs, often more than half an inch in length. With the European salmon, according to Mr. Lloyd,[9] the temporary hook-like structure serves to strengthen and protect the jaws, when one male charges another with wonderful violence; but the greatly developed teeth of the male American salmon may be compared with the tusks of many male mammals, and they indicate an offensive rather than a protective purpose.

{{centerFig 28 Head of female salmon.}}
The salmon is not the only fish in which the teeth differ in the two sexes; as this is the case with many rays. In the thornback (Raia clavata) the adult male has sharp, pointed teeth, directed backwards, whilst those of the female are broad and flat, and form a pavement; so that these teeth differ in the two sexes of the same species more than is usual in distinct genera of the same family. The teeth of the male become sharp only when he is adult: whilst young they are broad and flat like those of the female. As so frequently occurs with secondary sexual characters, both sexes of some species of rays (for instance R. batis), when adult, possess sharp pointed teeth; and here a character, proper to and primarily gained by the male, appears to have been transmitted to the offspring of both sexes. The teeth are likewise pointed in both sexes of R. maculata, but only when quite adult; the males acquiring them at an earlier age than the females. We shall hereafter meet with analogous cases in certain birds, in which the male acquires the plumage common to both sexes when adult, at a somewhat earlier age than does the female. With other species of rays the males even when old never possess sharp teeth, and consequently the adults of both sexes are provided with broad, flat teeth like those of the young, and like those of the mature females of the above-mentioned species.[10] As the rays are bold, strong and voracious fish, we may suspect that the males require their sharp teeth for fighting with their rivals; but as they possess many parts modified and adapted for the prehension of the female, it is possible that their teeth may be used for this purpose.
In regard to size, M. Carbonnier[11] maintains that the female of almost all fishes is larger than the male; and Dr. Günther does not know of a single instance in which the male is actually larger than the female. With some Cyprinodonts the male is not even half as large. As in many kinds of fishes the males habitually fight together, it is surprising that they have not generally become larger and stronger than the females through the effects of sexual selection. The males suffer from their small size, for according to M. Carbonnier, they are liable to be devoured by the females of their own species when carnivorous, and no doubt by other species. Increased size must be in some manner of more importance to the females, than strength and size are to the males for fighting with other males; and this perhaps is to allow of the production of a vast number of ova.
In many species the male alone is ornamented with bright colours; or these are much brighter in the male than the female. The male, also, is sometimes provided with appendages which appear to be of no more use to him for the ordinary purposes of life, than are the tail feathers to the peacock. I am indebted for most of the following facts to the kindness of Dr. Günther. There is reason to suspect that many tropical fishes differ sexually in colour and structure; and there are some striking cases with our British fishes. The male Callionymus lyra has been called the gemmeous dragonet "from its brilliant gem-like colours." When fresh caught from the sea the body is yellow of various shades, striped and spotted with vivid blue on the head; the dorsal fins are pale brown with dark longitudinal bands; the ventral, caudal, and anal fins being bluish-black. The female, or sordid dragonet, was considered by Linnæus, and by many subsequent naturalists, as a distinct species; it is of a dingy reddish-brown, with the dorsal fin brown and the other
N.B. The lower figure is more reduced than the upper.
The male of the Cottus scorpius, or sea-scorpion, is slenderer and smaller than the female. There is also a great difference in colour between them. It is difficult, as Mr. Lloyd[15] remarks, "for any one, who has not seen this fish during the spawning-season, when its hues are brightest, to conceive the admixture of brilliant colours with which it, in other respects so ill-favoured, is at that time adorned." Both sexes of the Labrus mixtus, although very different in colour, are beautiful; the male being orange with bright blue stripes, and the female bright red with some black spots on the back.
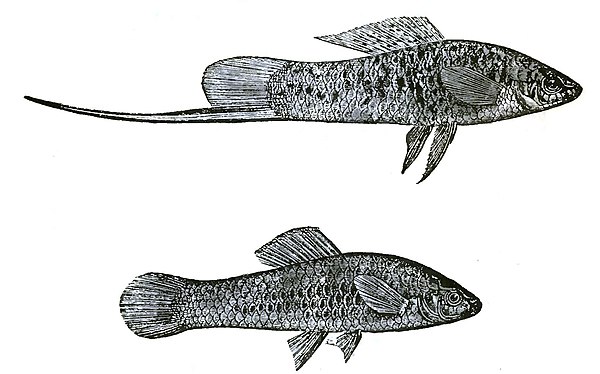
In the very distinct family of the Cyprinodontidæ—inhabitants of the fresh waters of foreign lands—the sexes sometimes differ much in various characters. In the male of the Mollienesia petenensis,[16] the dorsal fin is greatly developed and is marked with a row of large, round, ocellated, bright-coloured spots; whilst the same fin in the female is smaller, of a different shape, and marked only with irregularly curved brown spots. In the male the basal margin of the anal fin is also a little produced and dark coloured. In the male of an allied form, the Xiphophorus Hellerii (fig. 30), the inferior margin of the caudal fin is developed into a long filament, which, as I hear from Dr. Günther, is striped with bright colours. This filament does not contain any muscles, and apparently cannot be of any direct use to the fish. As in the case of the Callionymus, the males whilst young resemble the adult females in colour and structure. Sexual differences such as these may be strictly compared with those which are so frequent with gallinaceous birds.[17]
In a siluroid fish, inhabiting the fresh waters of South America, the Plecostomus barbatus[18] (fig. 31), the male has its mouth and inter-operculum fringed with a beard of stiff hairs, of which the female shows hardly a trace. These hairs are of the nature of scales. In another species of the same genus, soft flexible tentacles project from the front part of the head of the male, which are absent in the female. These tentacles are prolongations of the true skin, and therefore are not homologous with the stiff hairs of the former species; but it can hardly be doubted that both serve the same purpose. What this purpose may be, it is difficult to conjecture; ornament does not here seem probable, but we can hardly suppose that stiff hairs and flexible filaments can be useful in any ordinary way to the males alone. In that strange monster, the Chimæra monstrosa, the male has a hook-shaped bone on the top of the head, directed forwards, with its end rounded and covered with sharp spines; in the female "this crown is altogether absent," but what its use may be to the male is utterly unknown.[19]
The structures as yet referred to are permanent in the male after he has arrived at maturity; but with some Blennies, and in another allied genus,[20] a crest is developed on the head of the male only during the breeding-season, and the body at the same time becomes more brightly-coloured. There can be little doubt that this crest serves as a temporary sexual ornament, for the female does not exhibit a trace of it. In other species of the same genus both sexes possess a crest, and in at least one species neither sex is thus provided. In many of the Chromidæ, for instance in Geophagus and especially in Cichla, the males, as I hear from Professor Agassiz,[21] have a conspicuous protuberance on the forehead, which is wholly wanting in the females and in the young males. Professor Agassiz adds, "I have often observed these fishes at the time of spawning when the protuberance is largest, and at other seasons when it is totally wanting, and the two sexes shew no difference whatever in the outline of the profile of the head. I never could ascertain that it subserves any special function, and the Indians on the Amazon know nothing about its use." These protuberances resemble, in their periodical appearance, the fleshy caruncles on the heads of certain birds; but whether they serve as ornaments must remain at present doubtful.I hear from Professor Agassiz and Dr. Günther, that the males of those fishes, which differ permanently in colour from the females, often become more brilliant during the breeding-season. This is likewise the case with a multitude of fishes, the sexes of which are identical in colour at all other seasons of the year. The tench, roach, and perch may be given as instances. The male salmon at this season is "marked on the cheeks with orange-coloured stripes, which give it the appearance of a Labrus, and the body partakes of a golden orange tinge. The females are dark in colour, and are commonly called black-fish."[22] An analogous and even greater change takes place with the Salmo eriox or bull trout; the males of the char (S. umbla) are likewise at this season rather brighter in colour than the females.[23] The colours of the pike (Esox reticulatus) of the United States, especially of the male, become, during the breeding-season, exceedingly intense, brilliant, and iridescent.[24] Another striking instance out of many is afforded by the male stickleback (Gasterosteus leiurus), which is described by Mr. Warington,[25] as being then "beautiful beyond description." The back and eyes of the female are simply brown, and the belly white. The eyes of the male, on the other hand, are "of the most splendid green, having a metallic lustre like the green feathers of some humming-birds. The throat and belly are of a bright crimson, the back of an ashy-green, and the whole fish appears as though it were somewhat translucent and glowed with an internal incandescence." After the breeding-season these colours all change, the throat and belly become of a paler red, the back more green, and the glowing tints subside.
With respect to the courtship of fishes, other cases have been observed since the first edition of this book appeared, besides that already given of the stickleback. Mr. W. S. Kent says that the male of the Labrus mixtus, which, as we have seen, differs in colour from the female, makes "a deep hollow in the sand of the tank, and then endeavours in the most persuasive manner to induce a female of the same species to share it with him, swimming backwards and forwards between her and the completed nest, and plainly exhibiting the greatest anxiety for her to follow." The males of Cantharus lineatus become, during the breeding-season, of deep leaden-black; they then retire from the shoal, and excavate a hollow as a nest. "Each male now mounts vigilant guard over his respective hollow, and vigorously attacks and drives away any other fish of the same sex. Towards his companions of the opposite sex his conduct is far different; many of the latter are now distended with spawn, and these he endeavours by all the means in his power to lure singly to his prepared hollow, and there to deposit the myriad ova with which they are laden, which he then protects and guards with the greatest care.[26]
A more striking case of courtship, as well as of display, by the males of a Chinese Macropus has been given by M. Carbonnier, who carefully observed these fishes under confinement.[27] The males are most beautifully coloured, more so than the females. During the breeding-season they contend for the possession of the females; and, in the act of courtship, expand their fins, which are spotted and ornamented with brightly coloured rays, in the same manner, according to M. Carbonnier, as the peacock. They then also bound about the females with much vivacity, and appear by "l'étalage de leurs vives couleurs chercher à attirer l'attention des femelles, lesquelles ne paraissaient indifférentes à ce manége, elles nageaient avec une molle lenteur vers les mâles et semblaient se complaire dans leur voisinage." After the male has won his bride, he makes a little disc of froth by blowing air and mucus out of his mouth. He then collects the fertilised ova, dropped by the female, in his mouth; and this caused M. Carbonnier much alarm, as he thought that they were going to be devoured. But the male soon deposits them in the disc of froth, afterwards guarding them, repairing the froth, and taking care of the young when hatched. I mention these particulars because, as we shall presently see, there are fishes, the males of which hatch their eggs in their mouths; and those who do not believe in the principle of gradual evolution might ask how could such a habit have originated; but the difficulty is much diminished when we know that there are fishes which thus collect and carry the eggs; for if delayed by any cause in depositing them, the habit of hatching them in their mouths might have been acquired.
To return to our more immediate subject. The case stands thus: female fishes, as far as I can learn, never willingly spawn except in the presence of the males; and the males never fertilise the ova except in the presence of the females. The males fight for the possession of the females. In many species, the males whilst young resemble the females in colour; but when adult become much more brilliant, and retain their colours throughout life. In other species the males become brighter than the females and otherwise more highly ornamented, only during the season of love. The males sedulously court the females, and in one case, as we have seen, take pains in displaying their beauty before them. Can it be believed that they would thus act to no purpose during their courtship? And this would be the case, unless the females exert some choice and select those males which please or excite them most. If the female exerts such choice, all the above facts on the ornamentation of the males become at once intelligible by the aid of sexual selection.
We have next to enquire whether this view of the bright colours of certain male fishes having been acquired through sexual selection can, through the law of the equal transmission of characters to both sexes, be extended to those groups in which the males and females are brilliant in the same, or nearly the same degree and manner. In such a genus as Labrus, which includes some of the most splendid fishes in the world—for instance, the Peacock Labrus (L. pavo), described,[28] with pardonable exaggeration, as formed of polished scales of gold, encrusting lapis-lazuli, rubies, sapphires, emeralds, and amethysts—we may, with much probability, accept this belief; for we have seen that the sexes in at least one species of the genus differ greatly in colour. With some fishes, as with many of the lowest animals, splendid colours may be the direct result of the nature of their tissues and of the surrounding conditions, without the aid of selection of any kind. The gold-fish (Cyprinus auratus), judging from the analogy of the golden variety of the common carp, is perhaps a case in point, as it may owe its splendid colours to a single abrupt variation, due to the conditions to which this fish has been subjected under confinement. It is, however, more probable that these colours have been intensified through artificial selection, as this species has been carefully bred in China from a remote period.[29] Under natural conditions it does not seem probable that beings so highly organised as fishes, and which live under such complex relations, should become brilliantly coloured without suffering some evil or receiving some benefit from so great a change, and consequently without the intervention of natural selection.
What, then, are we to conclude in regard to the many fishes, both sexes of which are splendidly coloured? Mr. Wallace[30] believes that the species which frequent reefs, where corals and other brightly-coloured organisms abound, are brightly coloured in order to escape detection by their enemies; but according to my recollection they were thus rendered highly conspicuous. In the fresh-waters of the tropics there are no brilliantly-coloured corals or other organisms for the fishes to resemble; yet many species in the Amazons are beautifully coloured, and many of the carnivorous Cyprinidæ in India are ornamented with "bright longitudinal lines of various tints."[31] Mr. M'Clelland, in describing these fishes, goes so far as to suppose that the peculiar brilliancy of their colours" serves as "a better mark for king-fishers, terns, and other birds which are destined to keep the number of these fishes in check;" but at the present day few naturalists will admit that any animal has been made conspicuous as an aid to its own destruction. It is possible that certain fishes may have been rendered conspicuous in order to warn birds and beasts of prey that they were unpalatable, as explained when treating of caterpillars; but it is not, I believe, known that any fish, at least any fresh-water fish, is rejected from being distasteful to fish-devouring animals. On the whole, the most probable view in regard to the fishes, of which both sexes are brilliantly coloured, is that their colours were acquired by the males as a sexual ornament, and were transferred equally, or nearly so, to the other sex.
We have now to consider whether, when the male differs in a marked manner from the female in colour or in other ornaments, he alone has been modified, the variations being inherited by his male offspring alone; or whether the female has been specially modified and rendered inconspicuous for the sake of protection, such modifications being inherited only by the females. It is impossible to doubt that colour has been gained by many fishes as a protection: no one can examine the speckled upper surface of a flounder, and overlook its resemblance to the sandy bed of the sea on which it lives. Certain fishes, moreover, can through the action of the nervous system, change their colours in adaptation to surrounding objects, and that within a short time.[32] One of the most striking instances ever recorded of an animal being protected by its colour (as far as it can be judged of in preserved specimens), as well as by its form, is that given by Dr. Günther[33] of a pipe-fish, which, with its reddish streaming filaments, is hardly distinguishable from the sea-weed to which it clings with its prehensile tail. But the question now under consideration is whether the females alone have been modified for this object. We can see that one sex will not be modified through natural selection for the sake of protection more than the other, supposing both to vary, unless one sex is exposed for a longer period to danger, or has less power of escaping from such danger than the other; and it does not appear that with fishes the sexes differ in these respects. As far as there is any difference, the males, from being generally smaller and from wandering more about, are exposed to greater danger than the females; and yet, when the sexes differ, the males are almost always the more conspicuously coloured. The ova are fertilised immediately after being deposited; and when this process lasts for several days, as in the case of the salmon,[34] the female, during the whole time, is attended by the male. After the ova are fertilised they are, in most cases, left unprotected by both parents, so that the males and females, as far as oviposition is concerned, are equally exposed to danger, and both are equally important for the production of fertile ova; consequently the more or less brightly-coloured individuals of either sex would be equally liable to be destroyed or preserved, and both would have an equal influence on the colours of their offspring.
Certain fishes, belonging to several families, make nests, and some of them take care of their young when hatched. Both sexes of the bright coloured Crenilabrus massa and melops work together in building their nests with sea-weed, shells, &c.[35] But the males of certain fishes do all the work, and afterwards take exclusive charge of the young. This is the case with the dull-coloured gobies,[36] in which the sexes are not known to differ in colour, and likewise with the sticklebacks (Gasterosteus), in which the males become brilliantly coloured during the spawning season. The male of the smooth-tailed stickleback (G. leiurus) performs the duties of a nurse with exemplary care and vigilance during a long time, and is continually employed in gently leading back the young to the nest, when they stray too far. He courageously drives away all enemies, including the females of his own species. It would indeed be no small relief to the male, if the female, after depositing her eggs, were immediately devoured by some enemy, for he is forced incessantly to drive her from the nest.[37]
The males of certain other fishes inhabiting South America and Ceylon, belonging to two distinct Orders, have the extraordinary habit of hatching within their mouths or branchial cavities, the eggs laid by the females.[38] I am informed by Professor Agassiz that the males of the Amazonian species which follow this habit, "not only are generally brighter than the females, but the difference is greater at the spawning-season than at any other time." The species of Geophagus act in the same manner; and in this genus, a conspicuous protuberance becomes developed on the forehead of the males during the breeding-season. With the various species of Chromids, as Professor Agassiz likewise informs me, sexual differences in colour may be observed, "whether they lay their eggs in the water among aquatic plants, or deposit them in holes, leaving them to come out without further care, or build shallow nests in the river mud, over which they sit, as our Promotis does. It ought also to be observed that these sitters are among the brightest species in their respective families; for instance, Hygrogonus is bright green, with large black ocelli, encircled with the most brilliant red." Whether with all the species of Chromids it is the male alone which sits on the eggs is not known. It is, however, manifest that the fact of the eggs being protected or unprotected by the parents, has had little or no influence on the differences in colour between the sexes. It is further manifest, in all the cases in which the males take exclusive charge of the nests and young, that the destruction of the brighter-coloured males would be far more influential on the character of the race, than the destruction of the brighter-coloured females; for the death of the male during the period of incubation or nursing would entail the death of the young, so that they could not inherit his peculiarities; yet, in many of these very cases the males are more conspicuously coloured than the females.
In most of the Lophobranchii (Pipe-fish, Hippocampi, &c.) the males have either marsupial sacks or hemispherical depressions on the abdomen, in which the ova laid by the female are hatched. The males also shew great attachment to their young.[39] The sexes do not commonly differ much in colour; but Dr. Günther believes that the male Hippocampi are rather brighter than the females. The genus Solenostoma, however, offers a curious exceptional case,[40] for the female is much more vividly-coloured and spotted than the male, and she alone has a marsupial sack and hatches the eggs; so that the female of Solenostoma differs from all the other Lophobranchii in this latter respect, and from almost all other fishes, in being more brightly-coloured than the male. It is improbable that this remarkable double inversion of character in the female should be an accidental coincidence. As the males of several fishes, which take exclusive charge of the eggs and young, are more brightly coloured than the females, and as here the female Solenostoma takes the same charge and is brighter than the male, it might be argued that the conspicuous colours of that sex which is the more important of the two for the welfare of the offspring, must be in some manner protective. But from the large number of fishes, of which the males are either permanently or periodically brighter than the females, but whose life is not at all more important for the welfare of the species than that of the female, this view can hardly be maintained. When we treat of birds we shall meet with analogous cases, where there has been a complete inversion of the usual attributes of the two sexes, and we shall then give what appears to be the probable explanation, namely, that the males have selected the more attractive females, instead of the latter having selected, in accordance with the usual rule throughout the animal kingdom, the more attractive males.
On the whole we may conclude, that with most fishes, in which the sexes differ in colour or in other ornamental characters, the males originally varied, with their variations transmitted to the same sex, and accumulated through sexual selection by attracting or exciting the females. In many cases, however, such characters have been transferred, either partially or completely, to the females. In other cases, again, both sexes have been coloured alike for the sake of protection; but in no instance does it appear that the female alone has had her colours or other characters specially modified for this latter purpose.
The last point which need be noticed is that fishes are known to make various noises, some of which are described as being musical. Dr. Dufossé, who has especially attended to this subject, says that the sounds are voluntarily produced in several ways by different fishes: by the friction of the pharyngeal bones—by the vibration of certain muscles attached to the swim-bladder, which serves as a resounding board—and by the vibration of the intrinsic muscles of the swim-bladder. By this latter means the Trigla produces pure and long-drawn sounds which range over nearly an octave. But the most interesting case for us is that of two species of Ophidium, in which the males alone are provided with a sound-producing apparatus, consisting of small movable bones, with proper muscles, in connection with the swim-bladder.[41] The drumming of the Umbrinas in the European seas is said to be audible from a depth of twenty fathoms; and the fishermen of Rochelle assert "that the males alone make the noise during the spawning-time; and that it is possible by imitating it, to take them without bait."[42] From this statement, and more especially from the case of Ophidium, it is almost certain that in this, the lowest class of the Vertebrata, as with so many insects and spiders, sound-producing instruments have, at least in some cases, been developed through sexual selection, as a means for bringing the sexes together.
Amphibians.
Urodela.—I will begin with the tailed amphibians. The sexes of salamanders or newts often differ much both in colour and structure. In some species prehensile claws are developed on the fore-legs of the males during the breeding-season: and at this season in the male Triton palmipes the hind-feet are provided with a swimming-web, which is almost completely absorbed during the winter; so that their feet then resemble

Fig. 32. Triton cristatus (half natural size, from Bell's 'British Reptiles').
Upper figure, male during the breeding-season; lower figure, female.
those of the female.[43] This structure no doubt aids the male in his eager search and pursuit of the female. Whilst courting her he rapidly vibrates the end of his tail. With our common newts (Triton puncatus and crisatus) a deep, much indented crest is developed along the back and tail of the male during the breeding-season, which disappears during the winter. Mr. St. George Mivart informs me that it is not furnished with muscles, and therefore cannot be used for locomotion. As during the season of courtship it becomes edged with bright colours, there can hardly be a doubt that it is a masculine ornament. In many species the body presents strongly contrasted, though lurid tints, and these become more vivid during the breeding-season. The male, for instance, of our common little newt (Triton punctatus) is "brownish-grey above, passing into yellow beneath, which in the spring becomes a rich bright orange, marked everywhere with round dark spots." The edge of the crest also is then tipped with bright red or violet. The female is usually of a yellowish-brown colour with scattered brown dots, and the lower surface is often quite plain.[44] The young are obscurely tinted. The ova are fertilised during the act of deposition, and are not subsequently tended by either parent. We may therefore conclude that the males have acquired their strongly-marked colours and ornamental appendages through sexual selection; these being transmitted either to the male offspring alone, or to both sexes.
Anura or Butrachia.—With many frogs and toads the colours evidently serve as a protection, such as the bright green tints of tree-frogs and the obscure mottled shades of many terrestrial species. The most conspicuously-coloured toad which I ever saw, the Phryniscus nigricans,[45] had the whole upper surface of the body as black as ink, with the soles of the feet and parts of the abdomen spotted with the brightest vermilion. It crawled about the bare sandy or open grassy plains of La Plata under a scorching sun, and could not fail to catch the eye of every passing creature. These colours are probably beneficial by making this animal known to all birds of prey as a nauseous mouthful.
In Nicaragua there is a little frog "dressed in a bright livery of red and blue" which does not conceal itself like most other species, but hops about during the daytime, and Mr. Belt says[46] that as soon as he saw its happy sense of security, he felt sure that it was uneatable. After several trials he succeeded in tempting a young duck to snatch up a young one, but it was instantly rejected; and the duck "went about jerking its head, as if trying to throw off some unpleasant taste."
With respect to sexual differences of colour. Dr. Günther does not know of any striking instance either with frogs or toads; yet he can often distinguish the male from the female, by the tints of the former being a little more intense. Nor does he know of any striking difference in external structure between the sexes, excepting the prominences which become developed during the breeding-season on the front-legs of the male, by which he is enabled to hold the female.[47] It is surprising that these animals have not acquired more strongly-marked sexual characters; for though cold-blooded their passions are strong. Dr. Günther informs me that he has several times found an unfortunate female toad dead and smothered from having been so closely embraced by three or four males. Frogs have been observed by Professor Hoffman in Giessen fighting all day long during the breeding-season, and with so much violence, that one had its body ripped open.
Frogs and toads offer one interesting sexual difference, namely, in the musical powers possessed by the males; but to speak of music, when applied to the discordant and overwhelming sounds emitted by male bull-frogs and some other species, seems, according to our taste, a singularly inappropriate expression. Nevertheless, certain frogs sing in a decidedly pleasing manner. Near Rio Janeiro I used often to sit in the evening to listen to a number of little Hylæ, perched on blades of grass close to the water, which sent forth sweet chirping notes in harmony. The various sounds are emitted chiefly by the males during the breeding-season, as in the case of the croaking of our common frog.[48] In accordance with this fact the vocal organs of the males are more highly developed than those of the females. In some genera the males alone are provided with sacs which open into the larynx.[49] For instance, in the edible frog (Rana esculenta) "the sacs are peculiar to the males, and become, when filled with air in the act of croaking, large globular bladders, standing out one on each side of the head, near the corners of the mouth." The croak of the male is thus rendered exceedingly powerful; whilst that of the female is only a slight groaning noise.[50] In the several genera of the family the vocal organs differ considerably in structure, and their development in all cases may be attributed to sexual selection.
Reptiles.
Chelonia.—Tortoises and turtles do not offer well-marked sexual differences. In some species, the tail of the male is longer than that of the female. In some, the plastron or lower surface of the shell of the male is slightly concave in relation to the back of the female. The male of the mud-turtle of the United States (Chrysemys picta) has claws on its front-feet twice as long as those of the female; and these are used when the sexes unite.[51] With the huge tortoise of the Galapagos Islands (Testudo nigra) the males are said to grow to a larger size than the females: during the pairing-season, and at no other time, the male utters a hoarse bellowing noise, which can be heard at the distance of more than a hundred yards; the female, on the other hand, never uses her voice.[52]
With the Testudo elegans of India, it is said "that the combats of the males may be heard at some distance, from the noise they produce in butting against each other."[53]
Crocodilia.—The sexes apparently do not differ in colour; nor do I know that the males fight together, though this is probable, for some kinds make a prodigious display before the females. Bartram[54] describes the male alligator as striving to win the female by splashing and roaring in the midst of a lagoon, "swollen to an extent ready to burst, with its head and tail lifted up, he spins or twirls round on the surface of the water, like an Indian chief rehearsing his feats of war." During the season of love, a musky odour is emitted by the submaxillary glands of the crocodile, and pervades their haunts.[55]
Ophidia.—Dr. Günther informs me that the males are always smaller than the females, and generally have longer and slenderer tails; but he knows of no other difference in external structure. In regard to colour, he can almost always distinguish the male from the female by his more strongly-pronounced tints; thus the black zigzag band on the back of the male English viper is more distinctly defined than in the female. The difference is much plainer in the rattle-snakes of N. America, the male of which, as the keeper in the Zoological Gardens shewed me, can at once be distinguished from the female by having more lurid yellow about its whole body. In S. Africa the Bucephalus capensis presents an analogous difference, for the female "is never so fully variegated with yellow on the sides as the male."[56] The male of the Indian Dipsas cynodon, on the other hand, is blackish-brown, with the belly partly black, whilst the female is reddish or yellowish-olive, with the belly either uniform yellowish or marbled with black. In the Tragops dispar of the same country, the male is bright green, and the female bronze-coloured.[57] No doubt the colours of some snakes are protective, as shewn by the green tints of tree-snakes, and the various mottled shades of the species which live in sandy places; but it is doubtful whether the colours of many kinds, for instance of the common English snake and viper, serve to conceal them; and this is still more doubtful with the many foreign species which are coloured with extreme elegance. The colours of certain species are very different in the adult and young states.[58]
During the breeding-season the anal scent-glands of snakes are in active function;[59] and so it is with the same glands in lizards, and as we have seen with the submaxillary glands of crocodiles. As the males of most animals search for the females, these odoriferous glands probably serve to excite or charm the female, rather than to guide her to the spot where the male may be found. Male snakes, though appearing so sluggish, are amorous; for many have been observed crowding round the same female, and even round her dead body. They are not known to fight together from rivalry. Their intellectual powers are higher than might have been anticipated. In the Zoological Gardens they soon learn not to strike at the iron bar with which their cages are cleaned; and Dr. Keen of Philadelphia informs me that some snakes which he kept, learned after four or five times to avoid a noose, with which they were at first easily caught. An excellent observer in Ceylon, Mr. E. Layard, saw[60] a cobra thrust its head through a narrow hole and swallow a toad. "With this encumbrance he could not withdraw himself; finding this, he reluctantly disgorged the precious morsel, which began to move off; this was too much for snake philosophy to bear, and the toad was again seized, and again was the snake, after violent efforts to escape, compelled to part with its prey. This time, however, a lesson had been learnt, and the toad was seized by one leg, withdrawn, and then swallowed in triumph."
The keeper in the Zoological Gardens is positive that certain snakes, for instance Crotalus and Python, distinguish him from all other persons. Cobras kept together in the same cage apparently feel some attachment towards each other.[61]
It does not, however, follow because snakes have some reasoning power, strong passions and mutual affection, that they should likewise be endowed with sufficient taste to admire brilliant colours in their partners, so as to lead to the adornment of the species through sexual selection. Nevertheless, it is difficult to account in any other manner for the extreme beauty of certain species; for instance, of the coral-snakes of S. America, which are of a rich red with black and yellow transverse bands. I well remember how much surprise I felt at the beauty of the first coral-snake which I saw gliding across a path in Brazil. Snakes coloured in this peculiar manner, as Mr. Wallace states on the authority of Dr. Günther,[62] are found nowhere else in the world except in S. America, and here no less than four genera occur. One of these, Elaps, is venomous; a second and widely-distinct genus is doubtfully venomous, and the two others are quite harmless. The species belonging to these distinct genera inhabit the same districts, and are so like each other, that no one "but a naturalist would distinguish the harmless from the poisonous kinds." Hence, as Mr. Wallace believes, the innocuous kinds have probably acquired their colours as a protection, on the principle of imitation; for they would naturally be thought dangerous by their enemies. The cause, however, of the bright colours of the venomous Elaps remains to be explained, and this may perhaps be sexual selection.
Snakes produce other sounds besides hissing. The deadly Echis carinata has on its sides some oblique rows of scales of a peculiar structure with serrated edges; and when this snake is excited, these scales are rubbed against each other, which produces "a curious prolonged, almost hissing sound."[63] With respect to the rattling of the rattle-snake, we have at last some definite information: for Professor Aughey states,[64] that on two occasions, being himself unseen, he watched from a little distance, a rattle-snake coiled up with head erect, which continued to rattle at short intervals for half an hour: and at last he saw another snake approach, and when they met they paired. Hence he is satisfied that one of the uses of the rattle is to bring the sexes together. Unfortunately he did not ascertain whether it was the male or the female which remained stationary and called for the other. But it by no means follows from the above fact that the rattle may not be of use to these snakes in other ways, as a warning to animals which would otherwise attack them. Nor can I quite disbelieve the several accounts which have appeared of their thus paralysing their prey with fear. Some other snakes also make a distinct noise by rapidly vibrating their tails against the surrounding stalks of plants; and I have myself heard this in the case of a Trigonocephalus in S. America.
Lacertilia.—The males of some, probably of many kinds of lizards fight together from rivalry. Thus the arboreal Anolis cristatellus of S. America is extremely pugnacious: "During the spring and early part of the summer, two adult males rarely meet without a contest. On first seeing one another, they nod their heads up and down three or four times, and at the same time expanding the frill or pouch beneath the throat; their eyes glisten with rage, and after waving their tails from side to side for a few seconds, as if to gather energy, they dart at each other furiously, rolling over and over, and holding firmly with their teeth. The conflict generally ends in one of the combatants losing his tail, which is often devoured by the victor." The male of this species is considerably larger than the female;[65] and this, as far as Dr. Günther has been able to ascertain, is the general rule with lizards of all kinds. The males alone of the Cyrtodactylus rubidus of the Andaman Islands possesses pre-anal pores; and these pores judging from analogy probably serve to emit an odour.[66]
The sexes often differ greatly in various external characters. The male of the above-mentioned Anolis is furnished with a crest which runs along the back and tail, and can be erected at pleasure; but of this crest the female does not exhibit a trace. In the Indian Cophotis ceylanica, the female has a dorsal crest, though much less developed than in the male; and so it is, as Dr. Günther informs me, with the females of many Iguanas, Chameleons, and other lizards. In some species, however, the crest is equally developed in both sexes, as in the Iguana tuberculata. In the genus Sitana, the males alone are furnished with a large throat-pouch (fig. 33), which can be folded up like a fan, and is coloured blue, black, and red; but these splendid colours are exhibited only during the pairing-season. The female does not possess even a rudiment of this appendage. In the Anolis cristatellus, according to Mr. Austen, the throat pouch, which is bright red marbled with yellow, is present in the female, though in a rudimental condition. Again, in certain other lizards, both sexes are equally well provided with throat pouches. Here we see with species belonging to the same group, as in so many previous cases, the same character either confined to the males, or more largely developed in them than in the females, or again equally developed in both sexes. The little lizards of the genus Draco, which glide through the air on their rib-supported parachutes, and which in the beauty of their colours baffle description, are furnished with skinny appendages to the throat "like the wattles of gallinaceous birds." These become erected when the animal is excited. They occur in both sexes, but are best developed when the male arrives at maturity, at which age the middle appendage is sometimes twice as long as the head. Most of the species likewise have a low crest running along the neck; and this is much more developed in the full-grown males, than in the females or young males.[67] A Chinese species is said to live in pairs during the spring; "and if one is caught, the other falls from the tree to the ground, and allows itself to be captured with impunity,"—I presume from despair.[68]There are other and much more remarkable differences between the sexes of certain lizards. The male of Ceratophora aspera bears on the extremity of his snout an appendage half as long as the head. It is cylindrical, covered with scales, flexible, and apparently capable of erection: in the female it is quite rudimental. In a second species of the same genus a terminal scale forms a minute horn on the summit of the flexible appendage; and in a third species (C. Stoddartii, fig. 34) the whole appendage is converted into a horn, which is usually of a white colour, but assumes a purplish tint when the animal is excited. In the adult male of this latter species the horn is half an inch in length, but it is of quite minute size in the female and in the young. These appendages, as Dr. Günther has remarked to me, may be compared with the combs of gallinaceous birds, and apparently serve as ornaments.
In the genus Chamæleon we come to the acme of difference between the sexes. The upper part of the skull of the male C. bifurcus (fig. 35), an inhabitant of Madagascar, is produced into two great, solid, bony projections, covered with scales like the rest of the head; and of this wonderful modification of structure the female exhibits only a rudiment. Again, in Chamæleon Owenii (fig. 36), from the West Coast of Africa, the male bears on his snout and forehead three curious horns, of which the female has not a trace. These horns consist of an excrescence of bone covered with a smooth sheath, forming part of 
Fig 36. Chamæleon Owenii. Upper figure, male lower figure, female. the general integuments of the body, so that they are identical in structure with those of a bull, goat, or other sheath-horned ruminant. Although the three horns differ so much in appearance from the two great prolongations of the skull in C. bifurcus, we can hardly doubt that they serve the same general purpose in the economy of these two animals. The first conjecture, which will occur to every one, is that they are used by the males for fighting together; and as these animals are very quarrelsome,[69] this is probably a correct view. Mr. T. W. Wood also informs me that he once watched two individuals of C. pumilus, fighting violently on the branch of a tree; they flung their heads about and tried to bite each other; they then rested for a time, and afterwards continued their battle.
With many lizards, the sexes differ slightly in colour, the tints and stripes of the males being brighter and more distinctly defined, than in the females. This, for instance, is the case with the above Cophotis and with the Acanthodactylus capensis of S. Africa. In a Cordylus of the latter country, the male is either much redder or greener than the female. In the Indian Calotes nigrilabris there is a still greater difference; the lips also of the male are black, whilst those of the female are green. In our common little viviparous lizard (Zootoca vivipara) "the under side of the body and base of the tail in the male are bright orange, spotted with black; in the female these parts are pale-greyish-green without spots."[70] We have seen that the males alone of Sitana possess a throat-pouch; and this is splendidly tinted with blue, black, and red. In the Proctotretus tenuis of Chile the male alone is marked with spots of blue, green, and coppery-red.[71] In many cases the males retain the same colours throughout the year, but in others they become much brighter during the breeding-season; I may give as an additional instance the Calotes maria, which at this season has a bright red head, the rest of the body being green.[72]
Both sexes of many species are beautifully coloured exactly alike; and there is no reason to suppose that such colours are protective. No doubt with the bright green kinds which live in the midst of vegetation, this colour serves to conceal them; and in N. Patagonia I saw a lizard (Proctotretus multimaculatus) which, when frightened, flattened its body, closed its eyes, and then from its mottled tints was hardly distinguishable from the surrounding sand. But the bright colours with which so many lizards are ornamented, as well as their various curious appendages, were probably acquired by the males as an attraction, and then transmitted either to their male offspring alone, or to both sexes. Sexual selection, indeed, seems to have played almost as important a part with reptiles as with birds; and the less conspicuous colours of the females in comparison with the males cannot be accounted for, as Mr. Wallace believes to be the case with birds, by the greater exposure of the females to danger during incubation.
- ↑ Yarrell's 'Hist, of British Fishes,' vol. ii. 1836, pp. 417, 425, 436. Dr. Günther informs me that the spines in R. clacata are peculiar to the female.
- ↑ 'The American Naturalist,' April 1871, p. 119.
- ↑ See Mr. R. Warington's interesting articles in 'Annals and Mag. of Nat. Hist.' Oct. 1852 and Nov. 1855.
- ↑ Noel Humphreys, 'River Gardens,' 1857.
- ↑ Loudon's 'Mag. of Nat. History,' vol. iii. 1830, p. 331.
- ↑ 'The Field,' June 29th, 1867. For Mr. Shaw's statement, see 'Edinburgh Review,' 1843. Another experienced observer (Scrope's 'Days of Salmon Fishing,' p. 60) remarks that like the stag, the male would, if he could, keep all other males away.
- ↑ Yarrell, 'History of British Fishes,' vol. ii. 1836, p. 10.
- ↑ 'The Naturalist in Vancouver's Island,' vol. i. 1866, p. 54.
- ↑ 'Scandinavian Adventures,' vol i. 1854, pp. 100, 104.
- ↑ See Yarrell's account of the rays in his 'Hist. of British Fishes,' vol. ii. 1836, p. 410, with an excellent figure, and p. 422, 432.
- ↑ As quoted in 'The Farmer,' 1868, p. 369.
- ↑ I have drawn up this description from Yarrell's 'British Fishes,' vol. i. 1836, pp. 261 and 266.
- ↑ 'Nature,' July 1873, p. 264.
- ↑ 'Catalogue of Acanth. Fishes in the British Museum,' by Dr. Günther, 1861, pp. 138–151.
- ↑ 'Game Birds of Sweden,' &c., 1867, p. 466.
- ↑ With respect to this and the following species I am indebted to Dr. Günther for information: see also his paper on the 'Fishes of Central America,' in 'Transact. Zoolog. Soc.' vol. vi. 1868, p. 485.
- ↑ Dr. Günther makes this remark; 'Catalogue of Fishes in the British Museum,' vol. iii. 1861, p. 141.
- ↑ See Dr. Günther on this genus, in 'Proc. Zoolog. Soc.' 1868, p. 232.
- ↑ F. Buckland, in 'Land and Water,' July 1868, p. 377, with a figure. Many other cases could be added of structures peculiar to the male, of which the uses are not known.
- ↑ Dr. Günther, 'Catalogue of Fishes,' vol. iii. pp. 221 and 240.
- ↑ See also 'A Journey in Brazil,' by Prof. and Mrs. Agassiz, 1868, p. 220.
- ↑ Yarrell, 'British Fishes,' vol. ii. 1836, pp. 10, 12, 35.
- ↑ W. Thompson, in 'Annals and Mag. of Nat. History,' vol. vi. 1841, p. 440.
- ↑ 'The American Agriculturalist,' 1868, p. 100.
- ↑ 'Annals and Mag. of Nat. Hist.' Oct. 1852.
- ↑ 'Nature,' May, 1873, p. 25.
- ↑ 'Bull. de la Soc. d'Acclimat.' Paris, July 1869, and Jan. 1870.
- ↑ Bory de Saint Vincent, in 'Dict. Class. d'Hist. Nat.' tom. ix. 1826 p. 151.
- ↑ Owing to some remarks on this subject, made in my work 'On the Variation of Animals under Domestication,' Mr. W. F. Mayers ('Chinese Notes and Queries,' Aug. 1868, p. 123) has searched the ancient Chinese encyclopedias. He finds that gold-fish were first reared in confinement during the Sung Dynasty, which commenced A.D. 960. In the year 1129 these fishes abounded. In another place it is said that since the year 1548 there has been "produced at Hangchow a variety called the fire-fish, from its intensely red colour. It is universally admired, and there is not a household where it is not cultivated, in rivalry as to its colour, and as a source of profit."
- ↑ 'Westminster Review,' July 1867, p. 7.
- ↑ 'Indian Cyprinidæ,' by Mr. J. M'Clelland, 'Asiatic Researches,' vol. xix. part ii. 1839, p. 230.
- ↑ G. Pouchet, L'Institut. Nov. 1, 1871, p. 134.
- ↑ 'Proc. Zoolog. Soc.' 1865, p. 327, pl. xiv. and xv.
- ↑ Yarrell, 'British Fishes,' vol. ii. p. 11.
- ↑ According to the observations of M. Gerbe; see Günther's 'Record of Zoolog. Literature,' 1865, p. 194.
- ↑ Cuvier, 'Règne Animal,' vol. ii. 1829, p. 242.
- ↑ See Mr. Warington's most interesting description of the habits of the Gasterosteus leiurus, in 'Annals and Mag. of Nat. Hist.' November 1855.
- ↑ Prof. Wyman, in 'Proc. Boston Soc. of Nat. Hist.' Sept. 15, 1857. Also Prof. Turner, in 'Journal of Anatomy and Phys.' Nov. 1, 1866, p. 78. Dr. Günther has likewise described other cases.
- ↑ Yarrell, 'Hist, of British Fishes,' vol. ii. 1836, pp. 329, 338.
- ↑ Dr. Günther, since publishing an account of this species in 'The Fishes of Zanzibar,' by Col. Playfair, 1866, p. 137, has re-examined the specimens, and has given me the above information.
- ↑ 'Comptes Rendus.' Tom. xlvi. 1858, p. 353. Tom. xlvii. 1858, p. 916. Tom. liv. 1862, p. 393. The noise made by the Umbrinas (Sciœna aquila), is said by some authors to be more like that of a flute or organ, than drumming: Dr. Zouteveen, in the Dutch translation of this work (vol. ii., p. 36), gives some further particulars on the sounds made by fishes.
- ↑ The Rev. C. Kingsley, in 'Nature,' May 1870, p. 40.
- ↑ Bell, 'History of British Reptiles,' 2nd edit. 1849, pp. 156–159.
- ↑ Bell, 'History of British Reptiles,' 2nd edit. 1849, pp. 146, 151.
- ↑ 'Zoology of the Voyage of the "Beagle,"' 1843. Bell, ibid. p. 49.
- ↑ 'The Naturalist in Nicaragua,' 1874, p. 321.
- ↑ The male alone of the Bufo sikimmensis (Dr. Anderson, 'Proc. Zoolog. Soc.,' 1871, p. 204) has two plate-like callosities on the thorax and certain rugosities on the fingers, which perhaps subserve the same end as the above-mentioned prominences.
- ↑ Bell, 'History of British Reptiles,' 1849, p. 93.
- ↑ J. Bishop, in 'Todd's Cyclop. of Anat. and Phys.' vol. iv. p. 1503.
- ↑ Bell, ibid. p. 112–114.
- ↑ Mr. C. J. Maynard, 'The American Naturalist,' Dec. 1869, p. 555
- ↑ See my 'Journal of Researches during the Voyage of the " Beagle,"' 1845, p. 384.
- ↑ Dr. Günther, 'Reptiles of British India,' 1864, p. 7.
- ↑ 'Travels through Carolina,' &c., 1791, p. 128.
- ↑ Owen, 'Anatomy of Vertebrates,' vol. i. 1866, p. 615.
- ↑ Sir Andrew Smith, 'Zoolog. of S. Africa: Reptilia,' 1849, pl. x.
- ↑ Dr. A. Günther, 'Reptiles of British India,' Ray Soc. 1864, pp. 304, 308.
- ↑ Dr. Stoliczka, 'Journal of Asiatic Soc. of Bengal,' vol. xxxix. 1870, pp. 205, 211.
- ↑ Owen, 'Anatomy of Vertebrates,' vol. i. 1866, p. 615.
- ↑ 'Rambles in Ceylon.' in 'Annals and Mag. of Nat. Hist.' 2nd series, vol. ix. 1852, p. 333.
- ↑ Dr. Günther, 'Reptiles of British India,' 1864, p. 340.
- ↑ 'Westminster Review,' July 1st, 1867, p. 32.
- ↑ Dr. Anderson, 'Proc. Zoolog. Soc.' 1871, p. 196.
- ↑ 'The American Naturalist,' 1873, p. 85.
- ↑ Mr. N. L. Austen kept these animals alive for a considerable time; see 'Land and Water,' July 1867, p. 9.
- ↑ Stoliczka, 'Journal of Asiatic Soc. of Bengal,' vol. xxxiv. 1870, p. 166.
- ↑ All the foregoing statements and quotations, in regard to Cophotis, Sitana and Draco, as well as the following facts in regard to Ceratophora and Chamæleon, are from Dr. Günther himself, or from his magnificent work on the 'Reptiles of British India, Ray Soc. 1864, pp. 122, 130, 135.
- ↑ Mr. Swinhoe, 'Proc. Zoolog. Soc.' 1870, p. 240.
- ↑ Dr. Bucholz, ’Monatsbericht K. Preuss. Akad.’ Jtm. 1874, p. 78.
- ↑ Bell, ’History of British Reptiles,’ 2nd edit. 1849, p. 40.
- ↑ For Proctotretus see 'Zoology of the Voyage of the "Beagle:" Reptiles,' by Mr. Bell, p. 8. For the Lizards of S. Africa, see 'Zoology of S. Africa: Reptiles,' by Sir Andrew Smith, pl. 25 and 39. For the Indian Calotes, see 'Reptiles of British India,' by Dr. Günther, p. 143.
- ↑ Günther in 'Proc. Zoolog. Soc.' 1870, p. 778, with a coloured figure.