BRACHIOPODA, an important and well-defined but extremely isolated class of invertebrates. The group may be defined as follows: Sessile solitary Coelomata with bivalved shells usually of unequal size and arranged dorso-ventrally. The head is produced into ciliated arms bearing tentacles. They reproduce sexually, and with doubtful exceptions are of separate sexes.
The name Brachiopod (βραχίων, an arm, and πούς, ποδός, a foot) was proposed for the class by F. Cuvier in 1805, and by A. M. C. Dumeril in 1809, and has since been very extensively adopted. The division of the group into Ecardines (Inarticulata), with no hinge to the shell and with an alimentary canal open at both ends, and Testicardines (Articulata), with a hinge between the dorsal and ventral valves and with no anus, was proposed by Owen and has been adopted by nearly all authors. In a later scheme based on our increased knowledge of fossil forms, the Brachiopoda are divided into four primary groups (orders). This is given at the end of the article, but it must not be forgotten that the existing forms with an anus (Ecardines) differ markedly from the aproctous members of the group (Testicardines).

Figs. 1 – 11.—Various forms of Brachiopoda. | |
1. Magellania [Waldheimia] cranium. A, ventral, |
7. Leptaena transversalis. A, ventral, B, dorsal valve. |
The soft body of the Brachiopod is in all cases protected by a shell composed of two distinct valves; these valves are always, except in cases of malformation, equal-sided, but not equivalved. The valves are, consequently, essentially symmetrical, which is not the case with the Lamellibranchiata,—so much so, that certain Brachiopod shells were named Lampades, or lamp shells, by some early naturalists; but while such may bear a kind of resemblance to an antique Etruscan lamp, by far the larger number in no way resemble one. The shell is likewise most beautiful in its endless shapes and variations. In some species it is thin, semi-transparent and glassy, in others massive. Generally the shell is from a quarter of an inch to about 4 in. in size, but in certain species it attains nearly a foot in breadth by something less in length, as is the case with Productus giganteus. The valves are also in some species very unequal in their respective thickness, as may be seen in Productus (Daviesiella)[1] llangollensis, Davidsonia verneuilii, &c., and while the space allotted to the animal is very great in many species, as in Terebratula sphaeroidalis, it is very small in others belonging to Strophomena, Leptaena, Chonetes, &c. The ventral valve is usually the thickest, and in some forms is six or seven times as great as the opposite one. The outer surface of many of the species presents likewise the most exquisite sculpture, heightened by brilliant shades, or spots of green, red, yellow and bluish black. Traces of the original colour have also been preserved in some of the fossil forms; radiating bands of a reddish tint have been often seen in well-preserved examples of Terebratula (Dielasma) hastata, T. (Dielasma) sacculus, T. communis, T. biplicata, and of several others. Some specimens of T. carnea are of a beautiful pale pink colour when first removed from their matrix, and E. Deslongchamps has described the tint of several Jurassic species.
The valves are distinguished as dorsal and ventral. The ventral valve is usually the larger, and in many genera, such as Terebratula and Rhynchonella, has a prominent beak or umbo, with a circular or otherwise shaped foramen at or near its extremity, partly bounded by one or two plates, termed a deltidium. Through the foramen passes a peduncle, by which the animal is in many species attached to submarine objects during at least a portion of its existence. Other forms show no indication of ever having been attached, while some that had been moored by means of a peduncle during the early portion of their existence have become detached at a more advanced stage of life, the opening becoming gradually cicatrized, as is so often seen in Leptaena rhomboidalis, Orthisina anomala, &c. Lastly, some species adhere to submarine objects by a larger or smaller portion of their ventral valve, as is the case with many forms of Crania, Thecidium, Davidsonia, &c. Some Cranias are always attached by the whole surface of their lower or ventral valve, which models itself and fills up all the projections or depressions existing on either the rock, shell or coral to which it adhered. These irregularities are likewise, at times, reproduced on the upper or dorsal valve. Some species of Strophalosia and Productus seem also to have been moored during life to the sandy or muddy bottoms on which they lived, by the means of tubular spines often of considerable length. The interior of the shell varies very much according to families and genera. On the inner surface of both valves several well-defined muscular, vascular and ovarian impressions are observable; they form either indentations of greater or less size and depth, or occur as variously shaped projections. In the Trimerellidae, for example, some of the muscles are attached to a massive or vaulted platform situated in the medio-longitudinal region of the posterior half or umbonal portion of both valves. In addition to these, there exists in the interior of the dorsal valve of some genera a variously modified, thin, calcified, ribbon-shaped skeleton for the support of the ciliated arms, and the form of this ribbon serves as one of the chief generic characters of both recent and extinct forms. This brachial skeleton is more developed in some genera than in others. In certain forms, as in Terebratula and Terebratulina, it is short and simple, and attached to a small divided hinge-plate, the two riband-shaped lamina being bent upwards in the middle (fig. 15). The cardinal process is prominent, and on each side of the hinge-plate are situated the dental sockets; the loop in Terebratulina becomes annular in the adult by the union of its crural processes (fig. 16). In Magellania [Waldheimia] it is elongated and reflected; the hinge-plate large, with four depressions, under which originates a median septum, which extends more or less into the interior of the shell (figs. 13 and 14). In Terebratella the loop is attached to the hinge-plate and to the septum (fig. 17). In Megerlia it is three times attached, first to the hinge-plate, and then to the septum by processes from the diverging and reflected positions of the loop. In Magas the brachial skeleton is composed of an elevated longitudinal septum reaching from one valve to the other, to which are affixed two pairs of calcareous lamellae, the lower ones riband-shaped; attached first to the hinge-plate, they afterwards proceed by a gentle curve near to the anterior portion of the septum, to the sides of which they are affixed; the second pair originate on both sides of the upper edge of the septum, extending in the form of two triangular anchor-shaped lamellae (fig. 18). In Bouchardia the septum only is furnished with two short anchor-shaped lamellae. Many more modifications are observable in different groups of which the great family Terebratulidae is composed. In Thecidium (figs. 3,4) the interior of the dorsal valve is variously furrowed to receive the lophophore folded in two or more lobes.
In the family Spiriferidae there are two conical spires directed outwards, and nearly filling the cavity of the shell (fig. 5); while in Atrypa the broad spirally coiled lamellae are vertical, and directed toward the centre of the dorsal valve. In the Rhynchonellidae there are two short slender curved laminae, while in many genera and even families, such as the Productidae, Strophomenidae, Lingulidae, Discinidae, &c., there exists no calcified support for the labial appendages. The ventral valve in many of the genera is provided with two curved hinge-teeth, which fit into corresponding sockets in the opposite valve, so that the valves cannot be separated without breaking one of the teeth.
Each valve of the shell is lined by a mantle which contains prolongations of the body cavity. The outer surfaces of the mantle secrete the shell, which is of the nature of a cuticle impregnated by calcareous salts. These often have the form of prisms of calcite surrounded by a cuticular mesh work; the whole is nourished and kept alive by processes, which in Crania are branched; these perforate the shell and permit the access of the coelomic fluid throughout its substance. These canals are closed externally and are absent in Rhynchonella, where the amount of calcareous deposit is small. In Lingula the shell is composed of alternate layers of chitin and of phosphate of lime. The free edges of the mantle often bear chitinous bristles or setae which project beyond the shell. As in the case of the Lamellibranchiata, the shell of the adult is not a direct derivative of the youngest shell of the larva. The young Brachiopod in all its species is protected by an embryonic shell called the “protegulum,” which sometimes persists in the umbones of the adult shells but is more usually worn off. In all species it has the same shape, a shape which has been retained in the adult by the Lower Cambrian genus Iphidea.
The body of the Brachiopod usually occupies about the posterior half of the space within the shell. The anterior half of this space is lined by the inner wall of the mantle and is called the mantle cavity. This cavity lodges the arms, which are curved and coiled in different ways in different genera. The water which bears the oxygen for respiration and the minute organisms upon which the Brachiopod feeds is swept into the mantle cavity by the action of the cilia which cover the arms, and the eggs and excreta pass out into the same cavity. The mouth lies in the centre of the anterior wall of the body. Its two lips fusing together at the corners of the mouth are prolonged into the so-called arms. These arms, which together form the lophophore, may be, as in Cistella, applied flat to the inner surface of the dorsal mantle fold, but more usually they are raised free from the body like a pair of moustaches, and as they are usually far too long to lie straight in the mantle cavity, they are folded or coiled up. The brachial skeleton which in many cases supports the arms has been mentioned above.
A transverse section through the arm (fig. 22) shows that it consists of a stout base, composed of a very hyaline connective tissue not uncommon in the tissues of the Brachiopoda, which is traversed by certain canals whose nature is considered below under the section (The Body Cavity) devoted to the coelom. Anteriorly this base supports a gurrie or gutter, the pre-oral rim of which is formed by a simple lip, but the post-oral rim is composed of a closely set row of tentacles. These may number some thousands, and they are usually bent over and tend to form a closed cylinder of the gutter. Each of these tentacles (fig. 22) is hollow, and it contains a diverticulum from the coelom, a branch of the vascular system, a nerve and some muscle-fibres. Externally on two sides and on the inner surface the tentacles are ciliated, and the cilia are continued across the gutter to the lip and even on the outer surface of the latter. These cilia pass on any diatoms and other minute organism which come within their range of action to the capacious oval mouth, which appears as a mere deepening of the gutter in the middle line. In Terebratulina, Rhynchonella, Lingula, and possibly other genera, the arms can be unrolled and protruded from the opened shell; in this case the tentacles also straighten themselves and wave about in the water.
The Body Cavity.—The various internal organs of the brachiopod body, the alimentary canal and liver, the excretory organs, the heart, numerous muscles and the reproductive organs, are enclosed in a cavity called the body cavity, and since this cavity (i.) is derived from the archicoel and is from the first surrounded by meroblast, (ii.) communicates with the exterior through the nephridia or excretory organs, and (iii.) gives rise by the proliferation of the cells which line it to the ova and spermatoza, it is of the nature of a true coelom. The coelom then is a spacious chamber surrounding the alimentary canal, and is continued dorsally and ventrally into the sinuses of the mantle (fig. 21). Some of the endothelial cells lining the coelom are ciliated, the cilia keeping the corpusculated fluid contents in movement. Others of the endothelial cells show a great tendency to form muscle fibres. Besides this main coelomic cavity there are certain other spaces which F. Blochmann regards as coelomic, but it must be remembered that his interpretation rests largely on histological grounds, and at present embryological confirmation is wanting. These spaces are as follows:—(i.) the great arm-sinus; (ii.) the small arm-sinus together with the central sinus and the peri-oesophageal sinus, and in Discinisca and Lingula, and, to a less extent, in Crania, the lip-sinus; (iii.) certain portions of the general body cavity which in Crania are separated off and contain muscles, &c.; (iv.) the cavity of the stalk when such exists. The great arm-sinus of each side of the lophophore lies beneath the fold or lip which together with the tentacles forms the ciliated groove in which the mouth opens. These sinuses are completely shut off from all other cavities, they do not open into the main coelomic space nor into the small arm-sinus, nor does the right sinus communicate with the left. The small arm-sinus runs along the arms of the lophophore at the base of the tentacles, and gives off a blind diverticulum into each of these. This diverticulum contains the blood-vessel and muscle-fibres (fig. 22). In the region of the mouth where the two halves of the small arm-sinus approach one another they open into a central sinus lying beneath the oesophagus and partly walled in by the two halves of the ventral mesentery. This sinus is continued round the oesophagus as the peri-oesophageal sinus, and thus the whole complex of the small arm-sinus has the relations of the so-called vascular system of a Sipunculid. In Crania it is completely shut off from the main coelom, but in Lingula it communicates freely with this cavity. In Discinisca and Lingula there is further a lip-sinus or hollow system of channels which traverses the supporting tissue of the edge of the mantle and contains muscle-fibres. It opens into the peri-oesophageal sinus. It is better developed and more spacious in Lingula than in Discinisca. In Crania, where only indications of the lip-sinus occur, there are two other closed spaces. The posterior occlusor muscles lie in a special closed space which Blochmann also regards as coelomic. The posterior end of the intestine is similarly surrounded by a closed coelomic space known as the peri-anal sinus in which the rectum lies freely, unsupported by mesenteries. All these spaces contain a similar coagulable fluid with sparse corpuscles, and all are lined by ciliated cells. There is further a great tendency for the endothelial cells to form muscles, and this is especially pronounced in the small arm-sinus, where a conspicuous muscle is built up. The mantle-sinuses which form the chief spaces in the mantle are diverticula of the main coelomic cavity. In Discinisca they are provided with a muscular valve placed at their point of origin. They contain the same fluid as the general coelom. The stalk is an extension of the ventral body-wall, and contains a portion of the coelom which, in Discinisca and Lingula, remains in communication with the general body cavity.
The Alimentary Canal.— The mouth, which is quite devoid of armature, leads imperceptibly into a short and dorsally directed oesophagus. The latter enlarges into a spherical stomach into which open the broad ducts of the so-called liver. The stomach then passes into an intestine, which in the Testicardines (Articulata) is short, finger-shaped and closed, and in the Ecardines (Inarticulata) is longer, turned back upon its first course, and ends in an anus. In Lingula and Discina the anus lies to the right in the mantle-cavity, but in Crania it opens medianly into a posterior extension of the same. Apart from the asymmetry of the intestine caused by the lateral position of the anus in the two genera just named, Brachiopods are bilaterally symmetrical animals.
The liver consists of a right and left half, each opening by a broad duct into the stomach. Each half consists of many lobes which may branch, and the whole takes up a considerable proportion of the space in the body cavity. The food passes into these lobes, which may be found crowded with diatoms, and without doubt a large part of the digestion is carried on inside the liver. The stomach, oesophagus and intestine are ciliated on their inner surface. The intestine is slung by a median dorsal and ventral mesentery which divides the body cavity into two symmetrically shaped halves; it is “stayed” by two transverse septa, the anterior or gastroparietal band running from the stomach to the body wall and the posterior or ileoparietal band running from the intestine to the body wall. None of these septa is complete, and the various parts of the central body cavity freely communicate with one another. In Rhynchonella, where there are two pairs of kidneys, the internal opening of the anterior pair is supported by the gastroparietal band and that of the posterior pair by the ileoparietal band. The latter pair alone persists in all other genera.
The kidneys or nephridia open internally by wide funnel-shaped nephridiostomes and externally by small pores on each side of the mouth near the base of the arms. Each is short, gently curved and devoid of convolutions. They are lined by cells charged with a yellow or brown pigment, and besides their excretory functions they act as ducts through which the reproductive cells leave the body.
Circulatory System.—The structures formerly regarded as pseudohearts have been shown by Huxley to be nephridia; the true heart was described and figured by A. Hancock, but has in many cases escaped the observation of later zoologists. F. Blochmann in 1884, however, observed this organ in the living animal in species of the following genera:—Terebratulina, Magellania [Waldheimia], Rhynchonella, Megathyris (Argiope), Lingula, and Crania (fig. 21). It consists of a definite contractile sac or sacs lying on the dorsal side of the alimentary canal near the oesophagus, and in preparations of Terebratulina made by quickly removing the viscera and examining them in sea-water under a microscope, he was able to count the pulsations, which followed one another at intervals of 30–40 seconds.
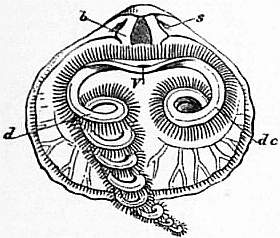 |
| Fig. 23.—Rhynchonella (Hemithyris) psittacea. Interior of dorsal valve, s, Sockets; b, dental plates; V, mouth; de, labial appendage in its natural position; d, appendage extended or unrolled. |
A vessel—the dorsal vessel—runs forward from the heart along the dorsal surface of the oesophagus. This vessel is nothing but a split between the right and left folds of the mesentery, and its cavity is thus a remnant of the blastocoel. A similar primitive arrangement is thought by F. Blochmann to obtain in the genital arteries. Anteriorly the dorsal vessel splits into a right and a left half, which enter the small arm-sinus and, running along it, give off a blind branch to each tentacle (fig. 21). The right and left halves are connected ventrally to the oesophagus by a short vessel which supplies these tentacles in the immediate neighbourhood of the mouth. There is thus a vascular ring around the oesophagus. The heart gives off posteriorly a second median vessel which divides almost at once into a right and a left half, each of which again divides into two vessels which run to the dorsal and ventral mantles respectively. The dorsal branch sends a blind twig into each of the diverticula of the dorsal mantle-sinus, the ventral branch supplies the nephridia and neighbouring parts before reaching the ventral lobe of the mantle. Both dorsal and ventral branches supply the generative organs.
The blood is a coagulable fluid. Whether it contains corpuscles is not yet determined, but if so they must be few in number. It is a remarkable fact that in Discinisca, although the vessels to the lophophore are arranged as in other Brachiopods, no trace of a heart or of the posterior vessels has as yet been discovered.
Muscles.—The number and position of the muscles differ materially in the two great divisions into which the Brachiopoda have been grouped, and to some extent also in the different genera of which each division is composed. Unfortunately almost every anatomist who has written on the muscles of the Brachiopoda has proposed different names for each muscle, and the confusion thence arising is much to be regretted. In the Testicardines, of which the genus Terebratula may be taken as an example, five or six pairs of muscles are stated by A. Hancock, Gratiolet and others to be connected with the opening and closing of the valves, or with their attachment to or movements upon the peduncle. First of all, the adductors or occlusors consist of two muscles, which, bifurcating near the centre of the shell cavity, produce a large quadruple impression on the internal surface of the small valve (fig. 13, a, a'), and a single divided one towards the centre of the large or ventral valve (fig. 12, a). The function of this pair of muscles is the closing of the valves. Two other pairs have been termed divaricators by Hancock, or cardinal muscles (“muscles diducteurs” of Gratiolet), and have for function the opening of the valves. The divaricators proper are stated by Hancock to arise from the ventral valve, one on each side, a little in advance of and close to the adductors, and after rapidly diminishing in size become attached to the cardinal process, a space or prominence between the sockets in the dorsal valve. The accessory divaricators are, according to the same authority, a pair of small muscles which have their ends attached to the ventral valve, one on each side of the median line, a little behind the united basis of the adductors, and again to the extreme point of the cardinal process. Two pairs of muscles, apparently connected with the peduncle and its limited movements, have been minutely described by Hancock as having one of their extremities attached to this organ. The dorsal adjusters are fixed to the ventral surface of the peduncle, and are again inserted into the hinge-plate in the smaller valve. The ventral adjusters are considered to pass from the inner extremity of the peduncle, and to become attached by one pair of their extremities to the ventral valve, one on each side and a little behind the expanded base of the divaricators. The function of these muscles, according to the same authority, is not only that of erecting the shell; they serve also to attach the peduncle to the shell, and thus effect the steadying of it upon the peduncle. By alternate contracting they can cause a slight rotation of the animal in its stalk.
Such is the general arrangement of the shell muscles in the division composing the articulated Brachiopoda, making allowance for certain unimportant modifications observable in the animals composing the different families and genera thereof. Owing to the strong and tight interlocking of the valves by the means of curved teeth and sockets, many species of Brachiopoda could open their valves but slightly. In some species, such as Thecidea, the animal could raise its dorsal valve at right angles to the plane of the ventral one (fig. 4).
In the Ecardines, of which Lingula and Discina may be quoted as examples, the myology is much more complicated. Of the shell or valvular muscles W. King makes out five pairs and an odd one, and individualizes their respective functions as follows:—Three pairs are lateral, having their members limited to the sides of the shell; one pair are transmedians, each member passing across the middle of the reverse side of the shell, while the odd muscle occupies the umbonal cavity. The central and umbonal muscles effect the direct opening and closing of the shell, the laterals enable the valves to move forward and backward on each other, and the transmedians allow the similar extremities (the rostral) of the valves to turn from each other to the right or the left on an axis subcentrically situated, that is, the medio-transverse region of the dorsal valve. It was long a matter in discussion whether the animal could displace its valves sideways when about to open its shell, but this has been actually observed by Professors K. Semper and E. S. Morse, who saw the animal perform the operation. They mention that it is never done suddenly or by jerks, as the valves are at first always pushed to one side several times and back again on each other, at the same time opening gradually in the transverse direction till they rest opposite to one another and widely apart. Those who have not seen the animal in life, or who did not believe in the possibility of the valves crossing each other with a slight obliquity, would not consent to appropriating any of its muscles to that purpose, and consequently attributed to all the lateral muscles the simple function of keeping the valves in an opposite position, or holding them adjusted. We have not only the observations of Semper and Morse, but the anatomical investigations of King, to confirm the sliding action or lateral divarication of the valves of Lingula.

Fig. 27.—Lingula analina Diagram showing the muscular system (After Hancock.)
The letters indicate the muscles as in figs. 25 and 26.
A, Dorsal, a, Alimentary tube.
B, Ventral valve. e, Heart.
p, Peduncle.z, Anal aperture.
In the Testicardines, where no such sliding action of the valves was necessary or possible, no muscles for such an object were required, consequently none took rise from the lateral portions of the valves as in Lingula; but in an extinct group, the Trimerellidae, which seems to be somewhat intermediate in character between the Ecardines and Testicardines, have been found certain scars, which appear to have been produced by rudimentary lateral muscles, but it is doubtful (considering the shells are furnished with teeth, though but rudely developed) whether such muscles enabled the valves, as in Lingula, to move forward and backward upon each other. Crania in life opens its valves by moving upon the straight hinge, without sliding the valve.
The nervous system of Brachiopods has, as a rule, maintained its primitive connexion with the external epithelium. In a few places it has sunk into the connective-tissue supporting layer beneath the ectoderm, but the chief centres still remain in the ectoderm, and the fibrils forming the nerves are for the most part at the base of the ectodermal cells. Above the oesophagus is a thin commissure which passes laterally into the chief arm-nerve. This latter includes in its course numerous ganglion cells, and forms, according to F. Blochmann, the immensely long drawn out supra-oesophageal ganglion. The chief arm-nerve traverses the lophophore, being situated between the great arm-sinus and the base of the lip (figs. 22 and 28); it gives off a branch to each tentacle, and these all anastomose at the base of the tentacles with the second nerve of the arm, the so-called secondary arm-nerve. Like the chief arm-nerve, this strand runs through the lophophore, parallel indeed with the former except near the middle line, where it passes ventrally to the oesophagus. The lophophore is supplied by yet a third nerve, the under arm-nerve, which is less clearly defined than the others, and resembles a moderate aggregation of the nerve fibrils, which seem everywhere to underlie the ectoderm, and which in a few cases are gathered up into nerves. The under arm-nerve, which lies between the small arm-sinus and the surface, supplies nerves to the muscles of both arm-sinuses (figs. 22 and 28). Medianly, it has its origin in the sub-oesophageal ganglion, which, like the supra-oesophageal, is drawn out laterally, though not to the same extent.

Fig. 28.—Diagram of nervous system of Crania; from the dorsal side. The nerves running to the dorsal parts are white, with black edges; those running to the ventral parts are solid black. Magnified. (After Blochmann.)
1. Oesophagus.
2. Supra-oesophageal commisure.
3. Circum-oesophageal commisure.
4. Under arm-nerve.
5. Great arm-sinus.
6. Small arm-sinus.
7. Tentacle.
8. Lip of lophophore.
9. Infra-oesophageal commisure.
10. Chief arm-nerve.
11. Secondary arm-nerve.
12. Nerves to tentacles.
13. Sub-oesophageal ganglion.
14. Dorsal lateral nerve.
15. Sub-oesophageal portion of the secondary
arm-nerve.
16. Median pallial nerve of dorsal lobe of mantle.
17. Anterior occlusor muscle.
18. Posterior occlusor muscle.
19. Obliquus superior muscle.
20. Levator brachii muscle.
In the middle line the sub-oespphageal nerve mass is small; the ganglion is in fact drawn out into two halves placed on either side of the body. From each of these sub-oesophageal ganglia numerous nerves arise. Passing from the middle line outwards they are—(i.) the median pallial nerve to the middle of the dorsal mantle; (ii.) numerous small nerves—the circum-oesophageal commissures—which pass round the oesophagus to the chief arm-nerve or supra-oesophageal ganglion; (iii.) the under arm-nerve to the lophophore and its muscles; (iv.) the lateral pallial nerve to the sides of the dorsal mantle. Laterally, the sub-oesophageal ganglia give off (v.) nerves to the ventral mantle, and finally they supply (vi.) branches to the various muscles. There is a special marginal nerve running round the edge of the mantle, but the connexion of this with the rest of the nervous system is not clear; probably it is merely another concentration of the diffused sub-ectodermal nervous fibrils.
The above account applies more particularly to Crania, but in the main it is applicable to the other Inarticulata which have been investigated. In Discinisca and Lingula, however, the sub-oesophageal ganglion is not drawn out, but lies medianly; it gives off two posteriorly directed nerves to the stalk, which in Lingula unite and form a substantial nerve. Sense organs are unknown in the adult. The larval forms are provided with eye-spots, but no very specialized sense organs are found in the adult.
The histology of Brachiopods presents some peculiar and many primitive features. As a rule the cells are minute, and this has especially stood in the way of embryological research. The plexus of nerve-fibrils which underlie the ectoderm and are in places gathered up into nerves, and the great development of connective tissue, are worthy of notice. Much of the latter takes the form of hyaline supporting tissue, embedded in which are scattered cells and fibres. The lophophore and stalk are largely composed of this tissue. The ectodermal cells are large, ciliated, and amongst the ciliated cells glandular cells are scattered. The chitinous chaetae have their origin in special ectodermal pits, at the base of which is one large cell which is thought to secrete the chaeta, as in Chaetopods. These pits are not isolated, but are connected by an ectodermal ridge, which grows in at the margin of the mantle and forms a continuous band somewhat resembling the ectodermal primordium of vertebrate teeth.
The ovary and testes are heaped-up masses of red or yellow cells due to a proliferation of the cells lining the coelom. There are four of such masses, two dorsal and two ventral, and as a rule they extend between the outer and inner layer of the mantle lining the shells. The ova and the spermatozoa dehisce into the body cavity and pass to the exterior through the nephridia. Fertilization takes place outside the body, and in some species the early stages of development take place in a brood-pouch which is essentially a more or less deep depression of the body-wall median in Thecidea, while in Cistella (? Argiope) there is one such pouch on each side, just below the base of the arms, and into these the nephridia open. The developing ova are attached by little stalks to the walls of these pouches. In spite of some assertions to the contrary, all the Brachiopods which have been carefully investigated have been found to be male or female. Hermaphrodite forms are unknown.
Embryology.—With the exception of Yatsu’s article on the development of Lingula (J. Coll. Sci., Japan, xvii., 1901 – 1903) and E. G. Conklin’s on “Terebratulina septentrionalis” (P. Amer. Phil. Soc. xli., 1902), little real advance has been made in our knowledge of the embryology of the Brachiopoda within recent years. Kovalevsky’s researches (Izv. Obshch. Moskov. xiv., 1874) on Megathyris (Argiope) and Yatsu’s just mentioned are the most complete as regards the earlier stages. Segmentation is complete, a gastrula is formed, the blastopore closes, the archenteron gives off two coelomic sacs which, as far as is known, are unaffected by the superficial segmentation of the body that divides the larva into three segments.
The walls of these sacs give rise at an early stage to muscles which enable the parts of the larva to move actively on one another (fig. 29, B). About this stage the larvae leave the brood-pouch, which is a lateral or median cavity in the body of the female, and lead a free swimming life in the ocean. The anterior segment broadens and becomes umbrella-shaped; it has a powerful row of cilia round the rim and smaller cilia on the general surface. By the aid of these cilia the larva swims actively, but owing to its minute size it covers very little distance, and this probably accounts for the fact that where brachiopods occur there are, as a rule, a good many in one spot. The head bears four eye-spots, and it is continually testing the ground (fig. 29, A, C). The second segment grows downwards like a skirt surrounding the third segment, which is destined to form the stalk.
It bears at its rim four bundles of very pronounced chaetae. After a certain time the larva fixes itself by its stalk to some stone or rock, and the skirt-like second segment turns forward over the head and forms the mantle. What goes on within the mantle is unknown, but presumably the head is absorbed. The chaetae drop off, and the lophophore is believed to arise from thickenings which appear in the dorsal mantle lobe. The Plankton Expedition brought back, and H. Simroth (Ergeb. Plankton Expedition, ii., 1897) has described, a few larval brachiopods of undetermined genera, two of which at least were pelagic, or at any rate taken far from the coast. These larvae, which resemble those described by Fritz Müller (Arch. Naturg., 1861 – 1862), have their mantle turned over their head and the larval shell well developed. No stalk has been seen by Simroth or Fritz Müller, but in other respects the larva resembles the stages in the development of Megathyris and Terebratulina which immediately precede fixation. The cirri or tentacles, of which three or four pairs are present, are capable of being protruded, and the minute larva swims by means of the ciliary action they produce. It can retract the tentacles, shut its shell, and sink to the bottom.
C. E. E. Beecher (Amer. Jour. Sci. ser. 3, xli. and xliv.) has classified with appropriate names the various stages through which Brachiopod larvae pass. The last stage, that in which the folds of the second segment are already reflected over the first, he calls the Typembryo. Either before or just after turning, the mantle develops a larval shell termed the protegulum, and when this is completed the larva is termed the Phylembryo. By this time the eyes have disappeared, the four bundles of chaetae have dropped off, and the lophophore has begun to appear as an outgrowth of the dorsal mantle lobe. The protegulum has been found in members of almost all the families of Brachiopod, and it is thought to occur throughout the group. It resembles the shell of the Cambrian genus Iphidea [Paterina], and the Phylembryo is frequently referred to as the Paterina stage. In some orders the Phylembryo is succeeded by an Obolella stage with a nearly circular outline, but this is not universal. The larva now assumes specific characters and is practically adult.
 |
| Fig. 31.—Shell of larval Brachiopod. Phylembryo stage. (From Simroth.) 1, Protegulum; 2, permanent shell. |
Classification.—Beecher’s division of the Brachiopoda into four orders is based largely on the character of the aperture through which the stalk or pedicle leaves the shell. To appreciate his diagnoses it is necessary to understand certain terms, which unfortunately are not used in the same sense by all authors. The triangular pedicle-opening seen in Orthis, &c., has been named by James Hall and J. M. Clarke the delthyrium. In some less primitive genera, e.g. Terebratula, that type of opening is found in the young stages only; later it becomes partly closed by two plates which grow out from the sides of the delthyrium. These plates are secreted by the ventral lobe of the mantle, and were named by von Buch in 1834 the “deltidium.” The form of the deltidium varies in different genera. The two plates may meet in the middle line, and leave only a small oval opening near the centre for the pedicle, as in Rhynchonella; or they may meet only near the base of the delthyrium forming the lower boundary of the circular pedicle-opening, as in Terebratula; or the right plate may remain quite distinct from the left plate, as in Terebratella. The pro-deltidium, a term introduced by Hall and Clarke, signifies a small embryonic plate originating on the dorsal side of the body. It subsequently becomes attached to the ventral valve, and develops into the pseudo-deltidium, in the Neotremata and the Protremata. The pseudo-deltidium (so named by Bronn in 1862) is a single plate which grows from the apex of the delthyrium downwards, and may completely close the aperture. The pseudo-deltidium is sometimes reabsorbed in the adult. In the Telotremata neither pro-deltidium nor pseudo-deltidium is known. In the Atremata the pro-deltidium does not become fixed to the ventral valve, and does not develop into a pseudo-deltidium. The American use of the term deltidium for the structure which Europeans call the pseudo-deltidium makes for confusion. The development of the brachial supports has been studied by Friele, Fischer and Oehlert. A summary of the results is given by Beecher (Trans. Connect. Acad. ix., 1893; reprinted in Studies in Evolution, 1901).
 |
| Fig. 32.—Diagram of the pedicle-opening of Rhynchonella. Magnified. |
|
1. Umbo of ventral valve. |
The orders Atremata and Neotremata are frequently grouped together, as the sub-class Inarticulata or Ecardines—the Tretenterata of Davidson—and the orders Protremata and Telotremata, as the Articulata or Testicardines— the Clistenterata of Davidson. The following scheme of classification is based on Beecher’s and Schubert’s. Recent families are printed in italic type.
Class I. Ecardines (Inarticulata)
ORDER I. Atremata (Beecher).—Inarticulate Brachiopoda, with the pedicle passing out between the umbones, the opening being shared by both valves. Pro-deltidium attached to dorsal valves. FAMILIES.—Paterinidae, Obolidae, Trimerellidae, Lingulellidae, Lingulidae, Ligulasmatidae.
ORDER II. Neotremata (Beecher).—More or less circular, cone-shaped, inarticulate Brachiopoda. The pedicle passes out at right angles to the plane of junction of the valves of the shell; the opening is confined to the ventral valve, and may take the form of a slit, or may be closed by the development of a special plate called the listrium, or by a pseudo-deltidium. Pro-deltidium attached to ventral valve. FAMILIES.—Acrotretidae, Siphonotretidae, Trematidae, Discinidae, Craniidae.
Class II. Testicardines (Articulata)
ORDER III. Protremata (Beecher).—Articulate Brachiopoda, with pedicle-opening restricted to ventral valve, and either open at the hinge line or more or less completely closed by a pseudo-deltidium, which may disappear in adult. The pro-deltidium originating on the dorsal surface later becomes anchylosed with the ventral valve. FAMILIES.—Kutorginidae, Eichwaldiidae, Billingsellidae, Strophomenidae, Thecidiidae, Productidae, Richthofenidae, Orthidae, Clitambonitidae, Syntrophiidae, Porambonitidae, Pentameridae.
ORDER IV. Telotremata (Beecher).—Articulate Brachiopoda, with the pedicle-opening, confined in later life to the ventral valve, and placed at the umbo or beneath it. Deltidium present, but no pro-deltidium. Lophophore supported by calcareous loops, &c. FAMILIES.—Protorhynchidae, Rhynchonellidae, Centronellidae, Terebratulidae, Stringocephalidae, Megalanteridae, Terebratellidae, Atrypidae, Spiriferidae, Athyridae.
Affinities.—Little light has been thrown on the affinities of the Brachiopoda by recent research, though speculation has not been wanting. Brachiopods have been at various times placed with the Mollusca, the Chaetopoda, the Chaetognatha, the Phoronidea, the Polyzoa, the Hemichordata, and the Urochordata. None of these alliances has borne close scrutiny. The suggestion to place Brachiopods with the Polyzoa, Phoronis, Rhabdopleura and Cephalodiscus, in the Phylum Podaxonia made in Ency. Brit. (vol. xix, ninth edition, pp. 440-441) has not met with acceptance, and until we have a fuller account of the embryology of some one form, preferably an Inarticulate, it is wiser to regard the group as a very isolated one. It may, however, be pointed out that Brachiopods seem to belong to that class of animal which commences life as a larva with three segments, and that tri-segmented larvae have been found now in several of the larger groups.
Distribution.—Brachiopods first appear in the Lower Cambrian, and reached their highest development in the Silurian, from which upwards of 2000 species are known, and were nearly as numerous in the Devonian period; at present they are represented by some 140 recent species. The following have been found in the British area, as defined by A. M. Norman, Terebratulina caput-serpentis L., Terebratula (Gwynia) capsula Jeff., Magellania (Macandrevia) cranium Müll., M. septigera Lovén, Terebratella spitzbergenensis Dav., Megathyris decollata Chemn., Cistella cistellula S. Wood, Cryptopora gnomon Jeff., Rhynchonella (Hemithyris) psittacea Gmel., Crania anomala Müll., and Discinisca atlantica King. About one-half the 120 existing species are found above the 100-fathoms line. Below 150 fathoms they are rare, but a few such as Terebratulina wyvillei are found down to 2000 fathoms. Lingula is essentially a very shallow water form. As a rule the genera of the northern hemisphere differ from those of the southern. A large number of specimens of a species are usually found together, since their only mode of spreading is during the ciliated larval stage, which although it swims vigorously can only cover a few millimetres an hour; still it may be carried some little distance by currents.
Undue stress is often laid on the fact that Lingula has come down to us apparently unchanged since Cambrian times, whilst Crania, and forms very closely resembling Discina and Rhynchonella, are found from the Ordovician strata onwards. The former statement is, however, true of animals from other classes at least as highly organized as Brachiopods, e.g. the Gasteropod Capulus, whilst most of the invertebrate classes were represented in the Ordovician by forms which do not differ from their existing representatives in any important respect.
A full bibliography of Brachiopoda (recent and fossil) is to be found in Davidson’s Monograph of British Fossil Brachiopods, Pal. Soc. Mon. vi., 1886. The Monograph on Recent Brachiopoda, by the same author, Tr. Linn. Soc. London, Zool. ser. ii. vol. iv., 1886–1888, must on no account be omitted. (A. E. S.)
- ↑ Subgenera are indicated by round, synonyms by square brackets.








