GREGARINES (mod. Lat. Gregarina, from gregarius, collecting in a flock or herd, grex) a large and abundant order of Sporozoa Ectospora, in which a very high degree of morphological specialization and cytological differentiation of the cell-body is frequently found. On the other hand, the life-cycle is, in general, fairly simple. Other principal characters which distinguish Gregarines from allied Sporozoan parasites are as follows:—The fully-grown adult (trophozoite) is always “free” in some internal cavity, i.e. it is extracellular; in nearly all cases prior to sporulation two Gregarines (associates) become attached to one another, forming a couple (syzygy), and are surrounded by a common cyst; inside the cyst the body of each associate becomes segmented up into a number of sexual elements (gametes, primary sporoblasts), which then conjugate in pairs; the resulting copula (zygote, definitive sporoblast) becomes usually a spore by the secretion of spore-membranes (sporocyst), its protoplasm (sporoplasm) dividing up to form the germs (sporozoites).
F. Redi (1684) is said to have been the first to observe a Gregarine parasite, but his claim to this honour is by no means certain. Much later (1787) Cavolini described and figured an indubitable Gregarine (probably the form now known as Aggregata conformis) from a Crustacean Historical.(Pachygrapsus), which, however, he regarded as a tapeworm. Leon Dufour, who in his researches on insect anatomy came across several species of these parasites, also considered them as allied to the worms and proposed the generic name of Gregarina. The unicellular nature of Gregarines was first realized by A. von Kölliker, who from 1845–1848 added considerably to our knowledge of the frequent occurrence and wide distribution of these organisms. Further progress was due to F. Stein who demonstrated about this time the relation of the “pseudo-navicellae” (spores) to the reproduction of the parasites.

|
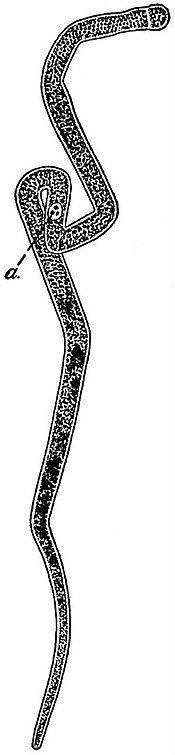
| From Wasielewswi’s Sporozoenkunde, after Pfeiffer. |
|
Fig. 1.—a, Transverse Section of Intestine of Mealworm, infected with Gregarina (Clepsydrina) polymorpha;[1] b, Part of a highly magnified. |
Apart from the continually increasing number of known species, matters remained at about this stage for many years. It is, in fact, only since the closing years of the 19th century that the complete life-history has been fully worked out; this has now been done in many cases, thanks to the researches of M. Siedlecki, L. Cuénot, L. Léger, O. Duboscq, A. Laveran, M. Caullery, F. Mesnil and others, to whom also we owe most of our knowledge regarding the relations of the parasites to the cells of their host during their early development.
Gregarines are essentially parasites of Invertebrates; they are not known to occur in any true Vertebrate although met with in Ascidians. By far the greatest number of hosts is furnished by the Arthropods. Many members of the various groups of worms (especially the Annelids) Occurrence; mode of infection. also harbour the parasites, and certain very interesting forms are found in Echinoderms; in the other classes, they either occur only sporadically or else are absent. Infection is invariably of the accidental (casual) type, by way of the alimentary canal, the spores being usually swallowed by the host when feeding; a novel variation of this method has been described by Woodcock (31) in the case of a Gregarine parasitic in Cucumaria, where the spores are sucked up through the cloaca into the respiratory trees, by the inhalant current.

|
| From Wasielewski, after Léger. |
|
Fig. 2.—Cysts of a Coelomic Gregarine, in the body-cavity of a larva of Tipula. |
The favourite habitat is either the intestine (fig. 1) or its diverticula (e.g. the Malpighian tubules), or the body-cavity. In the latter case, after infection has occurred, the liberated germs at once traverse the intestinal epithelium. They may come to rest in the connective tissue of the sub-mucosa (remaining, Habitat and effects on host. however, extracellular), grow considerably in that situation, and ultimately fall into the body-cavity (e.g. Diplocystis); or they may pass straightway into the body-cavity and there come into relation with some organ or tissue (e.g. Monocystis) of the earthworm, which is for a time intracellular in the spermatoblasts (fig. 4, c). In the case of intestinal Gregarines, the behaviour of the young trophozoite with respect to the epithelial cells of its host varies greatly. The parasite may remain only attached to the host-cell, never becoming actually intracellular (e.g. Pterocephalus); more usually it penetrates partially into it, the extracellular portion of the Gregarine, however, giving rise subsequently to most of the adult (e.g. Gregarina); or lastly, in a few forms, the early development is entirely intracellular (e.g. Lankesteria, Stenophora).
The effects on the host are confined to the parasitized cells. These generally undergo at first marked hypertrophy and alteration in character; this condition is succeeded by one of atrophy, when the substance of the cell becomes in one way or another practically absorbed by the growing parasite (cf. also Coccidia). Since, however, the Gregarines never overrun their hosts in the way that many other Sporozoa do (because of their lack, in general, of the power of endogenous multiplication), the number of cells of any tissue attacked, even in the case of a strong infection, is only a very small percentage of the whole. In short the hosts do not, as a rule, suffer any appreciable inconvenience from the presence of the parasites.
The body of a Gregarine is always of a definite shape, usually oval or elongated; in one or two instances (e.g. Diplodina) it is spherical, and, on the other hand, in Porospora (fig. 3) it is greatly drawn out and vermiform. In many adult Gregarines, the body is divided into two distinct but unequal regions Morphology. or halves, the anterior part being known as the protomerite, the hinder, generally the larger, as the deutomerite. This feature is closely associated with another important morphological character, one which is observable, however, only during the earlier stages of growth and development, namely, the presence of a definite organ, the epimerite, which serves for the attachment of the parasite to the host-cell (fig. 6).

|
| After Siedlecki, from Lankester’s Treatise on Zoology. |
| Fig. 5.—Part of a section through the apparatus of fixation of a Pterocephalus, showing root-like processes extending from the Gregarine between the epithelial cells. g, Head of Gregarine; r, Root-like processes; ep, Epithelial cells. |
In those Gregarines (most intestinal forms) which become attached to an epithelial cell, the attachment occurs by means of a minute projection or beak (rostrum) at the anterior end of the sporozoite, which pushes its way into the cell, followed by the first part of the growing germ. This portion of the body increases in size much quicker at first than the rest (the extracellular part), more or less fills up the host-cell, and forms the well-developed epimerite or secondary attaching organella. The extracellular part of the Gregarine next grows rapidly, and a transverse septum is formed at a short distance away from (outside) the point where the body penetrates into the cell (fig. 6); this marks off the large deutomerite posteriorly (distally). Léger thinks that this partition most likely owes its origin to trophic considerations, i.e. to the slightly different manner in which the two halves of the young parasite (the proximal, largely intracellular part, and the distal, extracellular one) may be supposed to obtain their nutriment. In the case of the one half, the host-cell supplies the nutriment, in that of the other, the intestinal liquid; and the septum is, as it were, the expression of the conflicting limit between these two methods. Nevertheless, the present writer does not think that mechanical considerations should be altogether left out of account. The septum may also be, to some extent, an adaption for strengthening the body of the fixed parasite against lateral thrusts or strains, due to the impact of foreign bodies (food, &c.) in the intestine.

|
| From Wasielewski, after Léger. |
| Fig. 6.—Corycella armata, Léger. a, Cephalont; b, Epimerite in host-cell; c, Sporont. |
At the point where the body becomes actually intracellular, it is constricted, and this constriction marks off the epimerite (internally) from the middle portion (between this point and the septum), which is the protomerite. Further growth is restricted, practically, to the extracellular regions, and the epimerite often comes to appear ultimately as a small appendage at the anterior end of the protomerite. A Gregarine at this stage is known as a cephalont. Later on, the parasite breaks loose from the host-cell and becomes free in the lumen, the separation taking place at the constriction between the protomerite and the epimerite; the latter is left behind in the remains of the host-cell, the former becomes the anterior part of the free trophozoite.
In other Gregarines, however, those, namely, which pass inwards, ultimately becoming “coelomic,” as well as those which become entirely intracellular, no epimerite is ever developed, and, further, the body remains single or unseptate. These forms, which include, for instance, Monocystis (fig. 4), Lankesteria, Diplocystis, are distinguished, as Acephalina or Aseptata (Haplocyta, Monocystida), according to which character is referred to, from the others, termed Cephalina or Septata (Polycystida).
The two sets of terms are not, however, completely identical or interchangeable, for there are a few forms which possess an epimerite, but which lack the division into protomerite and deutomerite, and are hence known as Pseudomonocystida; this condition may be primitive (Doliocystis) or (possibly) secondary, the partition having in course of time disappeared. Again, Stenophora is a septate form which has become, secondarily, completely intracellular during the young stages, and, doubtless correlated with this, shows no sign of an epimerite.

| ||
| From Wasielewski, after Léger. | ||
| Fig. 7.—Forms of Epimerites. | ||
| 1, Gregarina longa. | 6, Cometoides crinitus. | |
| 2, Sycia inopinata. | 7, Geneiorhynchus monnieri. | |
| 3, Pileocephalus heerii. | 8, Echinomera hispida. | |
| 4, Stylorhynchus longicollis. | 9, Pterocephalus nobilis. | |
| 5, Beloides firmus. | ||
With regard to the epimerites themselves, they are of all variety of form and shape and need not be described in detail (fig. 7). In one or two cases, however, another variety of attaching organella is met with. Thus in Pterocephalus, only the rostrum of the sporozoite penetrates into the host-cell, and no epimerite is formed. Instead, a number of fine root-like processes are developed from near the anterior end, which pass in between the host-cells (fig. 5) and thus anchor the parasite firmly. Similarly, in the curious Schizogregarinae, the anterior end of the (unseptate) body forms a number of stiff, irregular processes, which perform the same function (fig. 8). It is to be noted that these processes are non-motile, and not in any way comparable to pseudopodia, to which they were formerly likened.

|
| After Léger and Hagenmüller, from Lankester’s
Treatise on Zoology. |
| Fig. 8.—Three Individuals (G) of Ophryocystis schneideri, attached to wall of Malpighian tubule of Blaps sp. p, Syncytial protoplasm of the tubule; c, Cilia lining the lumen. |
A very interesting and remarkable morphological peculiarity has been recently described by Léger (18) in the case of a new Gregarine, Taeniocystis. In this form the body is elongated and metamerically segmented, recalling that of a segmented worm, the adult trophozoites possessing numerous partitions or segments (each corresponding to the septum between the proto- and deuto-merite in an ordinary Polycystid), which divide up the cytoplasm into roughly equal compartments. Léger thinks only the deutomerite becomes thus segmented, the protomerite remaining small and undivided. The nucleus remains single, so that there is no question as to the unicellular or individual nature of the entire animal.
The general cytoplasm usually consists of distinct ectoplasm and endoplasm, and is limited by a membrane or cuticle (epicyte), secreted by the former. The cuticle varies considerably in thickness, being well developed in active, intestinal forms, but very thin and delicate in non-motile coelomic Minute structure. forms (e.g. Diplodina). In the former case it may show longitudinal striations. The cuticle also forms the hooks or spines of many epimerites. The ectoplasm usually shows (fig. 9A) a differentiation into two layers, an outer, firmer layer, clear and hyaline, the sarcocyte, and an inner layer, the myocyte, which is formed of a network of muscle-fibrillae (mainly longitudinal and transverse, fig. 9B). The sarcocyte alone constitutes the septum, traversing the endoplasm, in septate Gregarines. The myonemes are undoubtedly the agents responsible for the active “gregarinoid” movements (of flexion and contraction) to be observed in many forms. The peculiar gliding movements were formerly thought to be produced by the extrusion of a gelatinous thread posteriorly, but Crawley (8) has recently ascribed them to a complicated succession of wave-like contractions of the myocyte layer. This view is supported by the fact that certain coelomic forms, like Diplodina and others, which either lack muscle-fibrils or else show no ectoplasmic differentiation at all, are non-motile. The endoplasm, or nutritive plasm, consists of a semi-fluid matrix in which are embedded vast numbers of grains and spherules of various kinds and of all sizes, representing an accumulation of food-material which is being stored up prior to reproduction. The largest and most abundant grains are of a substance termed para-glycogen, a carbohydrate; in addition, flattened lenticular platelets, of an albuminoid character, and highly-refringent granules often occur.
|
| |||||||||||||||||||||||||
The nucleus is always lodged in the endoplasm, and, in the septate forms, in the deutomeritic half of the body. It is normally spherical and always limited by a distinct nuclear membrane, which itself often contains chromatin. The most characteristic feature of the nucleus is the deeply-staining, more or less vacuolated spherical karyosome (consisting of chromatin intimately bound up with a plastinoid basis) which is invariably present. In one or two instances (e.g. Diplocystis schneideri) the nucleus has more than one karyosome. All the chromatin of the nucleus is not, however, confined to the karyosome, some being in the form of grains in the nuclear sap; and in some cases at any rate (e.g. Diplodina, Lankesteria) there is a well-marked nuclear reticulum which is impregnated with granules and dots of chromatin.

|
| From Wasielewski, after A. Schneider. |
|
Fig. 10.—Schizogony in Ophryocystis francisci. a, Rosette of small individuals, produced from a schizont which has just divided; b, A later stage, the daughter-individuals about to separate and assuming the characters of the adult. |
A sexual multiplication (schizogony) is only known certainly to occur in a few cases, one being in a Monocystid form, a species of Gonospora, which is for a long time intracellular (Caullery and Mesnil [4]), the rest among the Schizogregarinae, so named for this reason, in which schizogonous fission takes place regularly during Life-history.the free, trophic condition. Usually, the body divides up, by a process of multiple fission (fig. 10), into a few (up to eight) daughter-individuals; but in a new genus (Eleutheroschizon), Brasil (3) finds that a great number of little merozoites are formed, and a large amount of vacuolated cytoplasm is left over unused.
In the vast majority of Gregarines, however, the life-cycle is limited to gametogony and sporogony. A very general, if not indeed universal, prelude to gametogony is the characteristic and important feature of the order, known as association, the biological significance of which has only lately been fully brought out (see H. M. Woodcock [31]). In normal association, two individuals which are to be regarded as of opposite sex, come into close contact with each other and remain thus attached. The manner in which the parasites join varies in different forms; the association may be end-to-end (terminal), either by like or by unlike poles, or it may be side-to-side (lateral) (fig. 12). The couple (syzygy) thus formed may proceed forthwith to encystment and sporoblast-formation (Lankesteria, Monocystis), or may continue in the trophic phase for some time longer (Gregarina). In one or two instances (Zygocystis), association occurs as soon as the trophozoites become adult. This leads on to the interesting phenomenon of precocious association (neogamy), found in non-motile, coelomic Gregarines (e.g. Cystobia, Diplodina and Diplocystis), in which the parasitism is most advanced. Woodcock (loc. cit.) has described and compared the different methods adopted to ensure a permanent union, and the degree of neogamy attained, in these forms. Here it must suffice to say that, in the extreme condition (seen, for instance, in Diplodina minchinii) the union takes place very early in the life-history, between individuals which are little more than sporozoites, and is of a most intimate character, the actual cytoplasm of the two associates joining. In such cases, there is absolutely nothing to indicate the “double” nature of the growing trophozoite, but the presence of the two nuclei which remain quite distinct.

|
| From Wasielewski, after Léger. |
|
Fig. 11.—Eirmocystis spp. a, b, Associations of two and three Gregarines; c, Chain of five parasites; p, Primite; s, Satellites. |
There can be little doubt that, in the great majority, if not in all Gregarines, association is necessary for subsequent sporulation to take place; i.e. that the cytotactic attraction imparts a developmental stimulus to both partners, which is requisite for the formation of primary sporoblasts (gametes). This association is usually permanent; but in one or two cases (perhaps Gonospora sp.) temporary association may suffice. While association has fundamentally a reproductive (sexual) significance, in some cases, this function may be delayed or, as it were, temporarily suspended, the cytotactic attraction serving meanwhile a subsidiary purpose in trophic life. Thus, probably, are to be explained the curious multiple associations and long chains of Gregarines (fig. 11) sometimes met with (e.g. Eirmocystis, Clepsydrina).
Encystment is nearly always double, i.e. of an associated couple. Solitary encystment has been described, but whether successful independent sporulation results, is uncertain; if it does, the encystment in such cases is, in all probability, only after prior (temporary) association. In the case of free parasites, a well-developed cyst is secreted by the syzygy, which rotates and gradually becomes spherical. A thick, at first gelatinous, outer cyst-membrane (ectocyst) is laid down, and then a thin, but firm internal one (endocyst). The cyst once formed, further development is quite independent of the host, and, in fact, often proceeds outside it. In certain coelomic Gregarines, on the other hand, which remain in very close relation with the host’s tissues, little or nothing of an encystment-process on the part of the parasites is recognizable, the cyst-wall being formed by an enclosing layer of the host (Diplodina).

|
| From Wasielewski, after Léger. |
|
Fig. 12.—Associations of Gonospora sparsa. |
The nuclear changes and multiplication which precede sporoblast-formation vary greatly in different Gregarines and can only be outlined here. In the formation of both sets of sexual elements (gametes) there is always a comprehensive nuclear purification or maturation. This elimination of a part of the nuclear material (to be distinguished as trophic or somatic, from the functional or germinal portion, which forms the sexual nuclei) may occur at widely-different periods. In some cases (Lankesteria, Monocystis), a large part of the original (sporont-) nucleus of each associate is at once got rid of, and the resulting (segmentation-) nucleus, which is highly-specialized, represents the sexual part. In other cases, again, the entire sporont-nucleus proceeds to division, and the distinction between somatic and germinal portions does not become manifest until after nuclear multiplication has continued for some little time, when certain of the daughter-nuclei become altered in character, and ultimately degenerate, the remainder giving rise to the sporoblast-nuclei (Diplodina, Stylorhynchus). Even after the actual sporoblasts (sex-cells) themselves are constituted, their nuclei may yet undergo a final maturation (e.g. Clepsydrina ovata); and in Monocystis, indeed, Brasil (2) finds that what is apparently a similar process is delayed until after conjugation and formation of the zygote (definitive sporoblast).
Nuclear multiplication is usually indirect, the mitosis being, as a rule, more elaborate in the earlier than in the later divisions. The attraction-spheres are generally large and conspicuous, sometimes consisting of a well-developed centrosphere, with or without centrosomic granules, at other times of very large centrosomes with a few astral rays. In those cases where the karyosome is retained, and the sporont-nucleus divides up as a whole, however, the earliest nuclear divisions are direct; the daughter-nuclei being formed either by a process of simple constriction (e.g. Diplodina), or by a kind of multiple fission or fragmentation (Gregarina and Selenidium spp.). Nevertheless, the later divisions, at any rate in Diplodina, are indirect.
By the time nuclear multiplication is well advanced or completed, the bodies of the two parent-Gregarines (associates) have usually become very irregular in shape, and produced into numerous lobes and processes. While in some forms (e.g. Monocystis, Urospora, Stylorhynchus) the two individuals remain fairly separate and independent of each other, in others (Lankesteria) they become intertwined and interlocked, often to a remarkable extent (Diplodina). The sexual nuclei next pass to the surface of the processes and segments, where they take up a position of uniform distribution. Around each, a small area of cytoplasm becomes segregated, the whole often projecting as a little bud or hillock from the general surface. These uninuclear protuberances are at length cut off as the sporoblasts or gametes. Frequently a large amount of the general protoplasm of each parent-individual is left over unused, constituting two cystal residua, which may subsequently fuse; in Diplodina, however, practically the whole cytoplasm is used up in the formation of the gametes.
 | |
| After Léger, from Lankester’s Treatise on Zoology. | |
|
Fig. 13.—Development of the Gametes and Conjugation in Stylorhynchus longicollis. | |
| a, | Undifferentiated gamete, attached to body of parent-individual. |
| b-d, | Stages in development of motile male gamete. |
| e, | Mature female gamete. |
| f, g, | Stages in conjugation and nuclear union of the two elements. |
| h, | Zygote (copula). |
| i, | Spore, still with single nucleus and undivided sporoplasm. |
The sporoblasts themselves show all gradations from a condition of marked differentiation into male and female (anisogamy), to one of complete equality (isogamy). Anisogamy is most highly developed in Pterocephalus. Here, the male elements (microgametes) are minute, elongated and spindle-like in shape, with a minute rostrum anteriorly and a long flagellum posteriorly, and very active; the female elements (megagametes) are much larger, oblong to ovoid, and quite passive. In Stylorhynchus the difference between the conjugating gametes is not quite so pronounced (fig. 13), the male elements being of about the same bulk as the females, but pyriform instead of round, and possessing a distinct flagellum; a most interesting point about this parasite is that certain highly motile and spermatozoon-like male gametes are formed (fig. 13), which are, however, quite sterile and have acquired a subsidiary function. In other cases, again, the two kinds of element exhibit either very slight differences (Monocystis) or none (Urospora, Gonospora), in size and appearance, the chief distinction being in the nuclei, those of the male elements being smaller and chromatically denser than those of the females.
Lastly, in Lankesteria, Gregarina, Clepsydrina, Diplocystis and Diplodina complete isogamy is found, there being no apparent difference whatever between the conjugating elements. Nevertheless, these forms are also to be regarded as instances of binary sexuality and not merely of exogamy; for it is practically certain that this condition of isogamy is derived from one of typical anisogamy, through a stage such as is seen in Gonospora, &c. And, similarly, just as in all instances where the formation of differentiated gametes has been observed, the origin of the two conjugates is from different associates (parent-sporonts), and all the elements arising from the same parent are of the same sex, so it is doubtless the case here.
 |
|
Fig. 14.—Cyst of Monocystis agilis, the common Gregarine of the Earthworm, showing ripe spores and absence of any residual protoplasm in the cyst. (From Lankester.) |
The actual union is brought about or facilitated by the well-known phenomenon termed the danse des sporoblastes, which is due to various causes. In the case of highly-differentiated gametes (Pterocephalus), the actively motile microgametes rush about here and there, and seek out the female elements. In Stylorhynchus, Léger has shown that the function of the sterile male gametes is to bring about, by their vigorous movements, the mêlée sexuelle. In the forms where the gametes are isogamous or only slightly differentiated and (probably) not of themselves motile, other factors aid in producing the necessary commingling. Thus in Gregarina sp. from the mealworm, the unused somata or cystal residua become amoeboid and send out processes which drive the peripherally-situated gametes round in the cyst; in some cases where the residual soma becomes liquefied (Urospora) the movements of the host are considered to be sufficient; and lastly, in Diplodina, owing to the extent to which the intertwining process is carried, if each gamete is not actually contiguous to a suitable fellow-conjugant, a very slight movement or mutual attraction will bring two such, when liberated, into contact.
An unusual modification of the process of sporoblast-formation and conjugation, which occurs in Ophryocystis, must be mentioned. Here encystment of two associates takes place as usual; the sporont-nucleus of each, however, only divides twice, and one of the daughter-nuclei resulting from each division degenerates. Hence only one sporoblast-nucleus, representing a quarter of the original nuclear-material, persists in each half. Around this some of the cytoplasm condenses, the rest forming a residuum. The sporoblast or gamete thus formed is completely isogamous and normally conjugates with the like one from the other associate, when a single zygote results which becomes a spore containing eight sporozoites, in the ordinary manner. Sometimes, however, the septum between the two halves of the cyst does not break down, in which case parthenogenesis occurs, each sporoblast developing by itself into a small spore.
The two conjugating elements unite completely, cytoplasm with cytoplasm and nucleus with nucleus, to form the definitive sporoblast or zygote. The protoplasm assumes a definite outline, generally that of an ovoid or barrel, and secretes a delicate membrane, the ectospore. This subsequently becomes thickened, and often produced into rims, spines or processes, giving rise to the characteristic appearance of the Gregarine spore. Internal to the ectocyst, another, thinner membrane, the endocyst, is also laid down. These two membranes form the spore-wall (sporocyst). Meanwhile the contents of the spore have been undergoing division. By successive divisions, usually mitotic, the zygote-nucleus gives rise to eight daughter-nuclei, each of which becomes the nucleus of a sporozoite. Next, the sporoplasm becomes split longitudinally, around each nucleus, and thus eight sickle-shaped (falciform) sporozoites are formed. There is usually a certain amount of unused sporoplasm left over in the centre of the spore, constituting the sporal residuum. It is important to note that in all known Gregarines, with one exception, the number of sporozoites in the spore is eight; the exception is Selenidium, in many ways far from typical, where the number is half, viz. four.
 |
|
Fig. 15.—Ripe Cyst of Gregarina blattarum, partially emptied. (From Lankester.) a, Channels leading to the sporoducts; b, Mass of spores still left in the cyst; c, Endocyst; d, The everted sporoducts; e, Gelatinous ectocyst. |
Hitherto a variation from the general mode of spore-formation has been considered to occur in certain Crustacean Gregarines, the Aggregatidae and the Porosporidae. The spores of these forms have been regarded as gymnospores (naked), lacking the enveloping membranes (sporocyst) of the ordinary spores, and the sporozoites, consequently, as developed freely in the cyst. In the case of the first-named parasites, however, what was taken for sporogony has been proved to be really schizogony, and on other grounds these forms are, in the present writer’s opinion, preferably associated with the Coccidia (q.v.). With regard to the Porosporidae, also, it is quite likely that the gymnosporous cysts considered to belong to the Gregarine Porospora (as known in the trophic condition) have really no connexion with it, but represent the schizogonous generation of some other form, similar to Aggregata; in which case the true spores of Porospora have yet to be identified.
In the intestine of a fresh host the cysts rupture and the spores are liberated. This is usually largely brought about by the swelling of the residual protoplasm. Sometimes (e.g. Gregarina) long tubular outgrowths, known as sporoducts (fig. 15), are developed from the residual protoplasm, for the passage of the spores to the exterior.
The Gregarines are extremely numerous, and include several families, characterized, for the most part, by the form of the spores (fig. 16). The specialized Schizogregarinae are usually separated off from the rest as a distinct sub-order.Classification.
Sub-order I.—Schizogregarinae.
Forms in which schizogonic reproduction is of general occurrence during the extra-cellular, trophic phase. Three genera, Ophryocystis, Schizocystis and Eleutheroschizon, different peculiarities of which have been referred to above. Mostly parasitic in the intestine or Malpighian tubules of insects. (In this type of parasite, as exemplified by Ophryocystis, the body was formerly wrongly considered as amoeboid, and hence this genus was placed in a special order, the Amoebosporidia.)
 | ||
| From Wasielewski, after Léger. | ||
| Fig. 16.—Spores of various Gregarines. | ||
| a, Eirmocystis, Sphaerocystis, &c. | f, Stylorhynchidae (type of). | |
| b, Echinomera, Pterocephalus, &c. | g, Menosporidae. | |
| c, Gregarina, &c. | h, Gonospora terebellae. | |
| d, Beloides. | i, Ceratospora. | |
| e, Ancyrophora. | k, Urospora synaptae. | |
Sub-order II.—Eugregarinae.
Schizogony very exceptional, only occurring during the intracellular phase, if at all. Gregarines fall naturally into two tribes, described as cephalont and septate, or as acephalont and aseptate (haplocytic), respectively. In strictness, however, as already mentioned, these two sets of terms do not agree absolutely, and whichever set is adopted, the other must be taken into account in estimating the proper position of certain parasites. Here the cephalont or acephalont condition is regarded as the more primary and fundamental.
Tribe A.—Cephalina (practically equivalent to Septata).
Save exceptionally, the body possesses an epimerite, at any rate during the early stages of growth, and is typically septate. Mostly intestinal parasites of Arthropods.
The chief families, with representative genera, are as follows: Porosporidae, with Porospora gigantea, at present thought to be gymnosporous; Gregarinidae (Clepsydrinidae), with Gregarina, Clepsydrina, Eirmocystis, Hyalospora, Cmenidospora, Stenophora; Didymophyidae, with Didymophyes; Dactylophoridae, with Dactylophorus, Pterocephalus, Echinomera, Rhopalonia; Actinocephalidae with Actinocephalus, Pyxinia, Coleorhynchus, Stephanophora, Legeria, Stictospora, Pileocephalus, Sciadophora; Acanthosporidae with Acanthospora, Corycella, Cometoides; Menosporidae with Menospora, Hoplorhynchus; Stylorhynchidae, with Stylorhynchus, Lophocephalus; Doliocystidae with Doliocystis; and Taeniocystidae, with Taeniocystis. The curious genus Selenidium is somewhat apart.
Tribe B.—Acephalina (practically equivalent to Aseptata, Haplocyta).
The body never possesses an epimerite and is non-septate. Chiefly coelomic parasites of “worms,” Holothurians and insects.
The Aseptata have not been so completely arranged in families as the Septata. Léger has distinguished two well-marked ones, but the remaining genera still want classifying more in detail. Fam. Gonosporidae, with Gonospora, Diplodina; and Urosporidae, with Urosopora, Cystobia, Lithocystis, Ceratospora; the genera Monocystis, Diplocystis Lankesteria and Zygocystis probably constitute another; Pterospora and, again, Syncystis are distinct; lastly, certain forms, e.g. Zygosoma, Anchora (Anchorina), are incompletely known.
There remains for mention the remarkable parasite, recently described by J. Nusbaum (24) under the appropriate name of Schaudinnella henleae, which inhabits the intestine of Henlea leptodera. Briefly enumerated, the principal features in the life-cycle are as follows. The young trophozoites (aseptate) are attached to the intestinal cells, but practically entirely extracellular. Association is very primitive in character and indiscriminate; it takes place indifferently between individuals which will give rise to gametes of the same or opposite sex. Often it is only temporary; at other times it is multiple, several adults becoming more or less enclosed in a gelatinous investment. Nevertheless, in no case does true encystment occur, the sex-cells being developed practically free. The female gametes are large and egg-like; the males, minute and sickle-like, but with no flagellum and apparently non-motile. While many of the zygotes (“amphionts”) resulting from copulation pass out to the exterior, to infect a new host, others, possessing a more delicate investing-membrane, penetrate in between the intestinal cells, producing a further infection (auto-infection). Numerous sporozoites are formed in each zygote. It will be seen that Schaudinnella is a practically unique form. While, on the one hand, it recalls the Gregarines in many ways, on the other hand it differs widely from them in several characteristic features, being primitive in some respects, but highly specialized in others, so that it cannot be properly included in the order. Schaudinnella rather represents a primitive Ectosporan parasite, which has proceeded upon a line of its own, intermediate between the Gregarines and Coccidia.
Bibliography.—Among the important papers relating to Gregarines are the following: 1. A. Berndt, “Beitrag zur Kenntnis der . . . Gregarinen,” Arch. Protistenk. I, p. 375, 3 pls. (1902); 2. L. Brasil, “Recherches sur la reproduction des Grégarines monocystidées,” Arch. zool. exp. (4) 3, p. 17, pl. 2 (1905), and op. cit. 4, p. 69, 2 pls. (1905); 3. L. Brazil, “Eleutheroschizon duboscqi, parasite nouveau, &c.,” op. cit. (N. et R.) (4), p. xvii., 5 figs. (1906); 4. M. Caullery and F. Mesnil, “Sur une Grégarine . . . présentant . . . une phase de multiplication asporulée,” C.R. Ac. Sci. 126, p. 262 (1898); 5. M. Caullery and F. Mesnil, “Le Parasitisme intracellulaire des Grégarines,” op. cit. 132, p. 220 (1901); 6. M. Caullery and F. Mesnil, “Sur une mode particulière de division nucléaire chez les Grégarines,” Arch. anat. microsc. 3, p. 146, 1 pl. (1900); 7. M. Caullery and F. Mesnil, “Sur quelques parasites internes des Annélides,” Misc. biol. (Trav. Stat. Wimereux), 9, p. 80, 1 pl. (1899); 7a. J. Cecconi, “Sur l’Anchorina sagittata, &c.,” Arch. Protistenk. 6, p. 230, 2 pls. (1905); 8. H. Crawley, “Progressive Movement of Gregarines,” P. Ac. Philad. 54, p. 4, 2 pls. (1902), also op. cit. 57, p. 89 (1905); 9. H. Crawley, “List of the Polycystid Gregarines of the U.S.,” op. cit. 55, pp. 41, 632, 4 pls. (1903); 10. L. Cuénot, “Recherches sur l’évolution et la conjugaison des Grégarines,” Arch. biol. 17, p. 581, 4 pls. (1901); 11. A. Laveran and F. Mesnil, “Sur quelques particularités de l’évolution d’une Grégarine et la réaction de la cellule-hôte,” C.R. Soc. Biol. 52, p. 554, 9 figs. (1900); 12. L. Léger, “Recherches sur les Grégarines,” Tabl. zool. 3, p. i., 22 pls. (1892); 13. L. Léger, “Contribution à la connaissance des Sporozoaires, &c.,” Bull. Sci. France, 30, p. 240, 3 pls. (1897); 14. L. Léger, “Sur un nouveau Sporozoaire (Schizocystis), &c.,” C.R. Ac. Sci. 131, p. 722 (1900); 15. L. Léger, “La Reproduction sexuée chez les Ophryocystis,” t. c. p. 761 (1900); 16. L. Léger, “Sur une nouvelle Grégarine (Aggregata coelomica,), &c.” op. cit. 132, p. 1343 (1901); 17. L. Léger, “La Reproduction sexuée chez les Stylorhynchus,” Arch. Protistenk. 3, p. 304, 2 pls. (1904); 18. L. Léger, “Etude sur Taeniocystis mira (Léger), &c.,” op. cit. 7, p. 307, 2 pls. (1906); 19. L. Léger and O. Duboscq, “La Reproduction sexuée chez Pterocephalus,” Arch. zool. exp. (N. et R.) (4) 1, p. 141, 11 figs. (1903); 20. L. Léger and O. Duboscq, “Aggregata vagans, n. sp., &c.” t. c. p. 147, 6 figs. (1903); 21. L. Léger and O. Duboscq, “Les Grégarines et l’épithélium intestinal, &c.,” Arch. parasitol. 6, p. 377, 4 pls. (1902); 22. L. Léger and O. Duboscq, “Nouvelles Recherches sur les Grégarines, &c.,” Arch. Protistenk. 4, p. 335, 2 pls. (1904); 23. M. Lühe, “Bau und Entwickelung der Gregarinen,” t. c. p. 88, several figs. (1904); 24. J. Nusbaum, “Über die . . . Fortpflanzung einer . . . Gregarine, Schaudinnella henleae,” Zeit. wiss. Zool. 75, p. 281, pl. 22 (1903); 25. F. Paehler, “Über die Morphologie, Fortpflanzung . . . von Gregarina ovata,” Arch. Protistenk. 4, p. 64, 2 pls. (1904); 26. S. Prowazek, “Zur Entwickelung der Gregarinen,” op. cit., 1, p. 297, pl. 9 (1902); 27. A. Schneider (Various memoirs on Gregarines), Tabl. zool. 1 and 2 (1886–1892); 28. H. Schnitzler, “Über die Fortpflanzung von Clepsydrina ovata,” Arch. Protistenk. 6, p. 309, 2 pls. (1905); 29. M. Siedlecki, “Über die geschlechtliche Vermehrung der Monocystis ascidiae,” Bull. Ac. Cracovie, p. 515, 2 pls. (1900); 30. M. Siedlecki, “Contribution à l’étude des changements cellulaires provoquées par les Grégarines,” Arch. anat. microsc. 4, p. 87, 9 figs. (1901); 31. H. M. Woodcock, “The Life-Cycle of Cystobia irregularis, &c.,” Q.J.M. Sci. 50, p. 1. 6 pls. (1906). (H. M. Wo.)
- ↑ Figures 1, 2, 6, 7, 10, 11, 12 and 16 are redrawn from Wasielewski’s Sporozoenkunde, by permission of the author and of the publisher, Gustav Fischer, Jena.