POLLINATION, in botany, the transference of the pollen from the stamen to the receptive surface, or stigma, of the pistil of a flower. The great variety in the form, colour and scent of flowers (see Flower) is intimately associated with pollination which is effected by aid of wind, insects and other agencies. Pollen may be transferred to the stigma of the same flower—self-pollination (or autogamy), or to the stigma of another flower on the same plant or another plant of the same species—cross-pollination (or allogamy). Effective pollination may also occur between flowers of different species, or occasionally, as in the case of several orchids, of different genera—this is known as hybridization.
The method of pollination is to some extent governed by the distribution of the stamens and pistil. In the case of unisexual flowers, whether monoecious, that is, with staminate and pistillate flowers on one and the same plant, such as many of our native trees—oak, beech, birch, alder, &c., or dioecious with staminate and pistillate flowers on different plants, as in willows and poplars, cross pollination only is possible. In bisexual or hermaphrodite flowers, that is, those in which both stamens and pistil are present, though self-pollination might seem the obvious course, this is often prevented or hindered by various arrangements which favour cross-pollination. Thus the anthers and stigmas in any given flower are often mature at different times; this condition, which is known as dichogamy and was first pointed out by Sprengel, may be so well marked that the stigma has ceased to be receptive before the anthers open, or the anthers have withered before the stigma becomes receptive, when cross pollination only is possible, or the stages of maturity in the two organs are not so distinct, when self-pollination becomes possible later on. The flower is termed proterandrous or proterogynous according as anthers or stigmas mature first. The term homogamy is applied to the simultaneous maturity of stigma and anthers. Spontaneous self-pollination is rendered impossible in some homogamous flowers in consequence of the relative position of the anthers and stigma—this condition has been termed herkogamy. Flowers in which the relative position of the organs allows of spontaneous self-pollination may be all alike as regards length of style and stamens (homomorphy or homostyly), or differ in this respect (heteromorphy) the styles and stamens being of different lengths in different flowers (heterostyly) or the stamens only are of different lengths (heteranthery). Flowers which are closed at the time of maturity of anthers and stigmas are termed cleistogamous.
Self-pollination is effected in very various ways. In the simplest case the anthers are close to the stigmas, covering these with pollen when they open; this occurs in a number of small annual plants, also in Narcissus, Crocus, &c. In snowdrop and other pendulous flowers the anthers form a cone around the style and the pollen falls on to the underlying stigmas, or in erect flowers the pollen may fall on to the stigmas which lie directly beneath the opening anthers (e.g. Narthecium). In very many cases the pollen is carried to the stigma by elongation, curvature or some other movement of the filament, the style or stigma, or corolla or some other part of the flower, or by correlated movements of two or more parts. For instance, in many flowers the filaments are at first directed outwards so that self-pollination is not possible, but later incline towards the stigmas and pollinate them (e.g. numerous Saxifragaceae, Cruciferae and others), or the style, which first projects beyond the anthers, shortens later on so that the anthers come into contact with the stigmas (e.g. species of Cactaceae), or the style bends so that the stigma is brought within the range of the pollen (e.g. species of Oenothera, Epilobium, most Malvaceae, &c.). In Mirabilis Jalapa and others the filaments and style finally become intertwined, so that pollen is brought in contact with the stigma. Self-pollination frequently becomes possible towards the end of the life of a flower which during its earlier stages has been capable only of cross-pollination. This is associated with the fact, so ably demonstrated by Darwin, that, at any rate in a large number of cases, cross-pollination yields, better results, as measured by the number of seeds produced and the strength of the offspring, than self-pollination; the latter is, however, preferable to absence of pollination. In many cases pollen has no effect on the stigma of the same flower, the plants are self sterile, in other cases external pollen is more effective (pre-potent) than pollen from the same flower; but in a very large number of cases experiment has shown that there is little or no difference between the effects of external pollen and that from the same flower.
Cross-pollination may occur between two flowers on the same plant (geitonogamy) or between flowers on distinct plants (xenogamy). The former, which is a somewhat less favourable method than the latter, is effected by air-currents, insect agency, the actual contact between stigmas and anthers in neighbouring flowers, where, as in the family Compositae, flowers are closely crowded, or by the fall of the pollen from a higher on to the stigmas of a lower flower. Anton Kerner has shown that crowded inflorescences such as those of Compositae and Umbelliferae are especially adapted for geitonogamy. Xenogamy is of course the only possible method in diclinous plants; it is also the usual method in monoclinous plants, owing to the fact that stamens and carpels often mature at different times (dichogomy), the plants being proterandrous or proterogynous. Even in homogamous flowers cross-pollination is in a large proportion of cases the effective method, at any rate at first, owing to the relative position of anther and stigma or the fact that the plant is self-sterile.
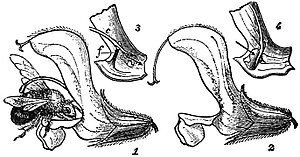
The subject of heterostyly was investigated by Darwin (see his Forms of Flowers) and later by Hildebrand. In the case of a dimorphic flower, such as Primula, four modes of pollination are possible, two distinguished by Darwin as legitimate, between anthers and stigmas on corresponding levels, and two so-called illegitimate unions, between anthers and stigmas at different levels (cf. fig. 1). In a trimorphic flower such as Lythrum salicaria there are six possible legitimate unions and twelve illegitimate (see fig. 2). Experiment showed that legitimate unions yield a larger quantity of seed than illegitimate.

|
|
Fig. 3.—Cleistogamous flower of Viola sylvatica. |
|
1, flower × 4. |
|
2, flower more highly magnified and cut open. a, anther; s, pistil; st, style; v, stigmatic surface. |
Many plants produce, in addition to ordinary open flowers, so-called cleistogamous flowers, which remain permanently closed but which notwithstanding produce fruit; in these the corolla is inconspicuous or absent and the pollen grows from the anther on to the stigma of the same flower. Species of Viola (see fig. 3), Oxalis acetosella (wood sorrel) and Lamium amplexicaule are commonly occurring instances. The cleistogamous flowers are developed before or after the normal open flowers at seasons less favourable for cross-pollination. In some cases flowers, which open under normal circumstances, remain closed owing to unfavourable circumstances, and self-pollination occurs as in a typical cleistogamous flower—these have been distingu1shed as pseudocleistogamous. Instances occur in water plants, where flowers are unable to reach the surface (e.g. Alisma natans, water buttercup, &c.) or where flowers remain closed in dull or cold weather.
Systems of classification of flowers according to the agency by which pollination is effected have been proposed by Delpino, H. Müller and other workers on the subject. Knuth suggests the following, which is a modification of the systems proposed by Delpino and Müller.
| A. | Water-pollinated plants, Hydrophilae. A small group which is subdivided thus: — | |||||||||||
| a. | Pollinated under the water; e.g. Najas where the pollen grains are rather heavier than water, and sinking down are caught by the stigmas of the extremely simple female flowers. | |||||||||||
| b. | Pollination on the surface, a more frequent occurrence than (a). In these the pollen floats on the surface and reaches the stigmas of the female flowers as in Callitriche, Ruppia, Zostera, Elodea. In Vallisneria (fig. 4) the male flowers become detached and float on the surface of the water; the anthers are thus brought in contact with the stigmas of the female flowers. | |||||||||||
| B. | Wind-pollinated plants, Anemophilae.—In these the pollen grains are smooth and light so as to be easily blown about, and are produced in great quantity; the stigmas are brush-like or feathery, and usually long and protruding so as readily to catch the pollen. As no means of attraction are required the flowers are inconspicuous and without scent or nectar. The male inflorescence is often a pendulous catkin, as in hazel and many native English trees (fig. 5); or the anthers are loosely fixed on long thread-like filaments as in grasses (fig. 6). | |||||||||||
| C. | Animal-pollinated plants, Zoidiophilae, are subdivided according to the kind of animal by agency of which pollination is effected, thus:— | |||||||||||
| a. | Bat-pollinated, Chiropterophilae.—A Freycinetia, native of Java, and a species of Bauhinia in Trinidad are visited by bats which transfer the pollen. | |||||||||||
| b. | Bird-pollinated, Ornithophilae.—Humming-birds and honey suckers are agents of pollination in certain tropical plants; they visit the generally large and brightly-coloured flowers either for the honey which is secreted in considerable quantity or for the insects which have been attracted by the honey (fig. 7). | |||||||||||
| ||||||||||||
| c. | Snail or slug-pollinated flowers, Malacophilae.—In small flowers which are crowded at the same level or in flat flowers in which the stigmas and anthers project but little, slugs or snails creeping over their surface may transfer to the stigma the pollen which clings to the slimy foot. Such a transfer has been described In Various Aroids, Rohdea japonica (Liliaceae), and other plants. | |||||||||||
| ||||||||||||
| d. |
| |||||||||||
| A. | Pollen Flowers.—These offer only pollen to their visitors, as species of anemone, poppy, rose, tulip, &c. They are simple in structure and regular in form, and the generally abundant pollen is usually freely exposed. | |||||||||||
| B. | Nectar Flowers.—These contain nectar and include the following groups:— | |||||||||||
| 1. | Flowers with exposed nectar, readily visible and accessible to all visitors. These are very simple, open and generally regular flowers, white, greenish-yellow or yellow in colour and are chiefly visited by insects with a short proboscis, such as short-tongued wasps and flies, also beetles and more rarely bees. Examples are Umbelliferae as a family, saxifrages, holly, Acer, Rhamnus, Euonymus, Euphorbia, &c. | |||||||||||
| 2. | Flowers with nectar partly concealed and visible only in bright sunshine. The generally regular flowers are completely open only in bright sunshine, closing up into cups at other times. Such are most Cruciferae, buttercups, king-cup (Caltha), Potentilla. White and yellow colours predominate and insects with a proboscis of medium length are the common pollinating agents, such as short-tongued bees. | |||||||||||
| 3. | Flowers with nectar concealed by pouches, hairs, &c. Regular flowers predominate, e.g. Geranium, Cardamine pratensis, mallows, Rubus, Oxalis, Epilobium, &c., but many species show more or less well-marked median symmetry (zygomorphism) as Euphrasia, Orchis, thyme, &c., and red, blue and violet are the usual colours. Long-tongued insects such as the honey-bee are the most frequent visitors. | |||||||||||
| 4. | Social flowers, whose nectar is concealed as in (3), but the flowers are grouped in heads which render them strikingly conspicuous, and several flowers can be simultaneously pollinated. Such are Compositae as a class, also Scabiosa, Armeria (sea-pink) and others. | |||||||||||
| 5. | Hymenopterid flowers, which fall into the following groups: Bee-flowers proper, humble-bee flowers requiring a longer proboscis to reach the nectar, wasp-flowers such as fig-wort (Scrophularia nodosa) and ichneumon flowers such as tway-blade (Listera ovata).
| |||||||||||
| In Broom there is an explosive mechanism; the pressure of the insect visitor on the keel of the corolla causes a sudden release of the stamens and the scattering of a cloud of pollen over its body. | ||||||||||||
| 6. | Lepidopterid flowers, visited chiefly by Lepidoptera, which are able to reach the nectar concealed in deep, narrow tubes or spurs by means of their long slender proboscis. Such are: (a) Butterfly-flowers, usually red in colour, as Dianthus carthusianorum; (b) Moth-flowers, white or whitish, as honeysuckle (Lonicera periclymenum). | |||||||||||
| 7. | Fly flowers, chiefly visited by Diptera, and including very different types:— | |||||||||||
| a. | Nauseous flowers, dull and yellowish and dark purple in colour and often spotted, with a smell attractive to carrion flies and dung flies, e.g. species of Saxifraga. | |||||||||||
| b. | Pitfall flowers such as Asarum, Aristolochia and Arum maculatum, when the insect is caught and detained until pollination is effected (fig. 10). | |||||||||||
| c. | Pinch-trap flowers, as in the family Asclepiadaceae, where the proboscis, claw or bristle of the insect is caught in the clip to which the pairs of pollinia are attached. Bees, wasps and larger insects serve as pollinating agents. | |||||||||||
| d. | Deceptive flowers such as Parnassia, where the conspicuous coronet of glistening yellow balls suggests a plentiful supply of nectar drops (fig. 11). | |||||||||||
| e. | Hoverfly flowers, small flowers which are beautifully coloured with radiating streaks pointing to a sharply-defined centre in which is the nectar, as in Veronica chamaedrys (fig. 12). | |||||||||||
 |
|
Fig. 11.—Grass of Parnassus (Parnassia palustris). |
|
1. One of the scales which form the coronet in the flower, enlarged. |
Literature.—Joseph Gottlieb Kölreuter[2] (d. 1806) was the first to study the pollination of flowers and to draw attention to the necessity of insect visits in many cases; he gave a clear account of cross-pollination by insect aid. He was followed by Christian Konrad Sprengel, whose work Das entdeckte Geheimniss der Natur im Bau und in der Befruchtung der Blumen (Berlin, 1793), contains a description of floral adaptations to insect visits in nearly 500 species of plants. Sprengel came very near to appreciating the meaning of cross-pollination in the life of plants when he states that “it seems that Nature is unwilling that any flower should be fertilized by its own pollen.” In 1799 an Englishman, Thomas Andrew Knight, after experiments on the cross-fertilization of cultivated plants, formulated the conclusion that no plant fertilizes itself through many generations. Sprengel’s work, which had been almost forgotten, was taken up again by Charles Darwin, who concluded that no organic being can fertilize itself through an unlimited number of generations; but a cross with other individuals is occasionally—perhaps at very long intervals—indispensable. Darwin’s works on dimorphic flowers and the fertilization of orchids gave powerful support to this statement. The study of the fertilization, or as it is now generally called “pollination,” of flowers, was continued by Darwin and taken up by other workers, notably Friedrich Hildebrand, Federico Delpino and the brothers Fritz and Hermann Müller. Hermann Müller’s work on The Fertilization of Flowers by Insects and their Reciprocal Adaptations (1873), followed by subsequent works on the same lines, brought together a great number of observations on floral mechanisms and their relation to insect-visits. Müller also suggested a modification of the Knight-Darwin law, which had left unexplained the numerous instances of continued successful self-pollination, and restated it on these terms: “Whenever offspring resulting from crossing comes into serious conflict with offspring resulting from self-fertilization, the former is victorious. Only where there is no such struggle for existence does self-fertilization often prove satisfactory for many generations.” An increasing number of workers in this field of plant biology in England, on the Continent and in America has produced a great mass of observations, which have recently been brought together in Dr Paul Knuth’s classic work, Handbook of Flower Pollination, an English translation of which has been published (1908) by the Clarendon Press.