dichotomously branched as in Macrozamia heteromera. In Ceratozamia
the broad petiole-base is characterized by the presence of two
lateral spinous processes, suggesting stipular appendages, comparable,
on a reduced scale, with the large stipules of the Marattiaceae
among Ferns. The vernation varies in different genera; in Cycas
the rachis is straight and the pinnae circinately coiled (fig. 3); in
Encephalartos, Dioon, &c., both rachis and segments are straight; in
Zamia the rachis is bent or slightly coiled, bearing straight pinnae.
The young leaves arise on the stem-apex as conical protuberances
with winged borders on which the pinnae appear as rounded humps,
usually in basipetal order; the scale-leaves in their young condition
resemble fronds, but the lamina remains undeveloped. A feature of
interest in connexion with the phylogeny of cycads is the presence of
long hairs clothing the scale-leaves, and forming a cap on the summit
of the stem-apex or attached to the bases of petioles; on some fossil
cycadean plants these outgrowths have the form of scales, and are
identical in structure with the ramenta (paleae) of the majority of ferns.
The male flowers of cycads are constructed on a uniform plan, and in all cases consist of an axis bearing crowded, spirally disposed sporophylls. These are often wedge-shaped and angular; in some cases they consist of a short, thick stalk, terminating in a peltate expansion, or prolonged upwards in Flower. the form of a triangular lamina. The sporangia (pollen-sacs), which occur on the under-side of the stamens, are often arranged in more or less definite groups or sori, interspersed with hairs (paraphyses); dehiscence takes place along a line marked out by the occurrence of smaller and thinner-walled cells bounded by larger and thicker-walled elements, which form a fairly prominent cap-like “annulus” near the apex of the sporangium, not unlike the annulus characteristic of the Schizaeaceae among ferns. The sporangial wall, consisting of several layers of cells, encloses a cavity containing numerous oval spores (pollen-grains). In structure a cycadean sporangium recalls those of certain ferns (Marattiaceae, Osmundaceae and Schizaeaceae), but in the development of the spores there are certain peculiarities not met with among the Vascular Cryptogams. With the exception of Cycas, the female flowers are also in the form of cones, bearing numerous carpellary scales. In Cycas revoluta and C. circinalis each leaf-like carpel may produce several laterally attached ovules, but in C. Normanbyana the carpel is shorter and the ovules are reduced to two; this latter type brings us nearer to the carpels of Dioon, in which the flower has the form of a cone, and the distal end of the carpels is longer and more leaf-like than in the other genera of the Zamieae, which are characterized by shorter carpels with thick peltate heads bearing two ovules on the morphologically lower surface. The cones of cycads attain in some cases (e.g. Encephalartos) a considerable size, reaching a length of more than a foot. Cases have been recorded (by Thiselton-Dyer in Encephalartos and by Wieland in Zamia) in which the short carpellary cone-scales exhibit a foliaceous form. It is interesting that no monstrous cycadean cone has been described in which ovuliferous and staminate appendages are borne on the same axis: in the Bennettitales (see Palaeobotany: Mesozoic) flowers were produced bearing on the same axis both androecium and gynoecium.

|
|
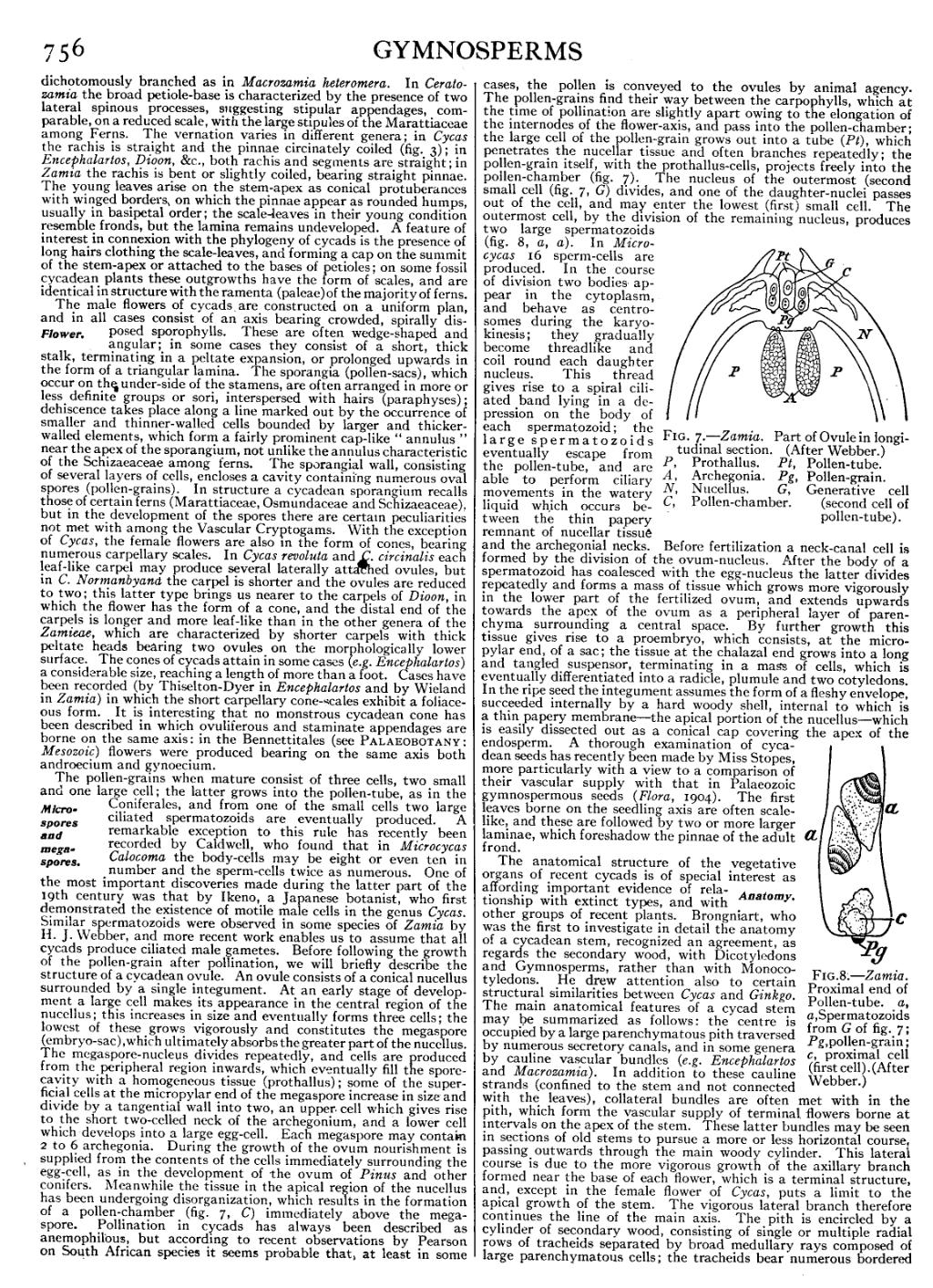
Fig. 8.—Zamia. Proximal end of Pollen-tube, a, a, Spermatozoids from G of fig. 7; Pg, pollen-grain; c, proximal cell (first cell). (After Webber.) |
The pollen-grains when mature consist of three cells, two small and one large cell; the latter grows into the pollen-tube, as in the Coniferales, and from one of the small cells two large ciliated spermatozoids are eventually produced. A remarkable exception to this rule has recently been Microspores and megaspores. recorded by Caldwell, who found that in Microcycas Calocoma the body-cells may be eight or even ten in number and the sperm-cells twice as numerous. One of the most important discoveries made during the latter part of the 19th century was that by Ikeno, a Japanese botanist, who first demonstrated the existence of motile male cells in the genus Cycas. Similar spermatozoids were observed in some species of Zamia by H. J. Webber, and more recent work enables us to assume that all cycads produce ciliated male gametes. Before following the growth of the pollen-grain after pollination, we will briefly describe the structure of a cycadean ovule. An ovule consists of a conical nucellus surrounded by a single integument. At an early stage of development a large cell makes its appearance in the central region of the nucellus; this increases in size and eventually forms three cells; the lowest of these grows vigorously and constitutes the megaspore (embryo-sac), which ultimately absorbs the greater part of the nucellus. The megaspore-nucleus divides repeatedly, and cells are produced from the peripheral region inwards, which eventually fill the spore-cavity with a homogeneous tissue (prothallus); some of the superficial cells at the micropylar end of the megaspore increase in size and divide by a tangential wall into two, an upper cell which gives rise to the short two-celled neck of the archegonium, and a lower cell which develops into a large egg-cell. Each megaspore may contain 2 to 6 archegonia. During the growth of the ovum nourishment is supplied from the contents of the cells immediately surrounding the egg-cell, as in the development of the ovum of Pinus and other conifers. Meanwhile the tissue in the apical region of the nucellus has been undergoing disorganization, which results in the formation of a pollen-chamber (fig. 7, C) immediately above the megaspore. Pollination in cycads has always been described as anemophilous, but according to recent observations by Pearson on South African species it seems probable that, at least in some cases, the pollen is conveyed to the ovules by animal agency. The pollen-grains find their way between the carpophylls, which at the time of pollination are slightly apart owing to the elongation of the internodes of the flower-axis, and pass into the pollen-chamber; the large cell of the pollen-grain grows out into a tube (Pt), which penetrates the nucellar tissue and often branches repeatedly; the pollen-grain itself, with the prothallus-cells, projects freely into the pollen-chamber (fig. 7). The nucleus of the outermost (second) small cell (fig. 7, G) divides, and one of the daughter-nuclei passes out of the cell, and may enter the lowest (first) small cell. The outermost cell, by the division of the remaining nucleus, produces two large spermatozoids (fig. 8, a, a). In Microcycas 16 sperm-cells are produced. In the course of division two bodies appear in the cytoplasm, and behave as centrosomes during the karyokinesis; they gradually become threadlike and coil round each daughter nucleus. This thread gives rise to a spiral ciliated band lying in a depression on the body of each spermatozoid; the large spermatozoids eventually escape from the pollen-tube, and are able to perform ciliary movements in the watery liquid which occurs between the thin papery remnant of nucellar tissue and the archegonial necks. Before fertilization a neck-canal cell is formed by the division of the ovum-nucleus. After the body of a spermatozoid has coalesced with the egg-nucleus the latter divides repeatedly and forms a mass of tissue which grows more vigorously in the lower part of the fertilized ovum, and extends upwards towards the apex of the ovum as a peripheral layer of parenchyma surrounding a central space. By further growth this tissue gives rise to a proembryo, which consists, at the micropylar end, of a sac; the tissue at the chalazal end grows into a long and tangled suspensor, terminating in a mass of cells, which is eventually differentiated into a radicle, plumule and two cotyledons. In the ripe seed the integument assumes the form of a fleshy envelope, succeeded internally by a hard woody shell, internal to which is a thin papery membrane—the apical portion of the nucellus—which is easily dissected out as a conical cap covering the apex of the endosperm. A thorough examination of cycadean seeds has recently been made by Miss Stopes, more particularly with a view to a comparison of their vascular supply with that in Palaeozoic gymnospermous seeds (Flora, 1904). The first leaves borne on the seedling axis are often scale-like, and these are followed by two or more larger laminae, which foreshadow the pinnae of the adult frond.
The anatomical structure of the vegetative organs of recent cycads is of special interest as affording important evidence of relationship with extinct types, and with other groups of recent plants. Brongniart, who was the first to investigate in detail the anatomy of a cycadean stem, recognized an agreement, as Anatomy. regards the secondary wood, with Dicotyledons and Gymnosperms, rather than with Monocotyledons. He drew attention also to certain structural similarities between Cycas and Ginkgo. The main anatomical features of a cycad stem may be summarized as follows: the centre is occupied by a large parenchymatous pith traversed by numerous secretory canals, and in some genera by cauline vascular bundles (e.g. Encephalartos and Macrozamia). In addition to these cauline strands (confined to the stem and not connected with the leaves), collateral bundles are often met with in the pith, which form the vascular supply of terminal flowers borne at intervals on the apex of the stem. These latter bundles may be seen in sections of old stems to pursue a more or less horizontal course, passing outwards through the main woody cylinder. This lateral course is due to the more vigorous growth of the axillary branch formed near the base of each flower, which is a terminal structure, and, except in the female flower of Cycas, puts a limit to the apical growth of the stem. The vigorous lateral branch therefore continues the line of the main axis. The pith is encircled by a cylinder of secondary wood, consisting of single or multiple radial rows of tracheids separated by broad medullary rays composed of large parenchymatous cells; the tracheids bear numerous bordered