Popular Science Monthly/Volume 59/October 1901/The Inert Constituents of the Atmosphere
| THE INERT CONSTITUENTS OF THE ATMOSPHERE. |
By Professor W. RAMSAY,
UNIVERSITY COLLEGE, LONDON.
THE discovery of an element always awakens interest; for the total number of the known elements does not exceed seventy-five, and all the various forms of matter which exist on this globe are necessarily composed of these elements. An element, as is well known, is the ultimate constituent of a compound; and with only a limited number, Nature has provided us with that enormous wealth of minerals, of vegetables and of animals, all of which have as their constituents two or more of these elements.
These elements, however, must not be regarded as isolated entities, each self-dependent, having no relations with its compeers; on the contrary, all the elements exhibit certain connections with their neighbors; and there is to be traced an orderly progression from one class of elements, strongly electro-positive in character, metallic in appearance, very inflammable when heated in the air, and at once attacked by water, to another class, highly electro-negative, transparent, unattackable by oxygen, and without perceptible action on water, through a number of connecting links, each of which serves to soften the transition.
These elements have been arranged in series, and it is by considering the method of arrangement that our interest is awakened. The earliest attempt to make such an arrangement antedates the very idea of the conception of an element. For the division of all matter into metal and non-metal is one which is lost in the mists of antiquity. The word 'metal' is derived from the Greek verb μεταλλάω, I search; and that verb is said to be derived from μέτα and ἅλλα, signifying 'after other things.' As it was recognized that elements are constituents of more complex matter, a conception first emphasized by Boyle, and as the distinction became clear that matter which resists decomposition must be classed as elementary and, after a century and a half, a number of elements were recognized, it was obvious that a number of them might be grouped in classes. Take, for example, the elements chlorine, bromine and iodine, all colored, strongly smelling substances, sparingly soluble in water and forming compounds barely distinguishable from each other in appearance or by a cursory inspection; or take such a group as the metals of the alkalies, lithium. sodium, potassium, rubidium and cæsium, all white, soft metals, all easily oxidizable, all at once violently attacked by water, and generally with such energy as to be inflamed at the contact. It required no great penetration to class such elements as these into classes.
The revival of the hypothesis of the atomic constitution of matter by Dalton and of his attempt to determine the atomic weights of the elements was not long in provoking the guess that perhaps there could be found some connection between the numbers representing the relative atomic weights of kindred elements. But, as is well known, the state of knowledge in Dalton's day was not sufficiently advanced to enable him to attribute to elements their correct relative atomic weights; and it was not until the eminent professor of chemistry in Rome, Cannizzaro, whose jubilee has recently been celebrated, pointed out the bearing on Dalton's numbers of all the facts accumulated up to the year 1856 that the close relationship between the atomic weights and the properties of the elements was suggested by John Newlands. Some years later, Lothar Meyer and Dmitri Mendeléef amplified and elaborated the ideas which had first been propounded by Newlands; and the periodicity of the atomic weights and the gradual variation of the properties of the elements and their compounds were established on a firm basis.
Various plans have been adopted to render this arrangement pictorially visible; each method has perhaps its own conveniences, but none can be regarded as the method par excellence. Lothar Meyer's original table is constructed on the hypothesis that a cylinder, on which the numbers have been distributed in their order on a descending spiral in eight main columns, has been unrolled.
Another method of representation is due to Dr. Johnstone Stoney. The atomic weights are represented on a spiral curve, closely approximating in form to a logarithmic spiral, and the magnitudes of the atomic weights are represented by the volumes of concentric spheres. Thus, the sphere in the middle stands for unity, the atomic weight of hydrogen. The elements follow each other according to the numerical order of their atomic weights; and by joining the points thus obtained, a nearly regular spiral curve is produced, resembling one derived by aid of a logarithmic or elliptical formula. The deviations from regularity appear also to follow a law, and if accurately mapped the spiral is a sinuous one. But the determination of individual atomic weights is as yet not sufficiently accurate to make it possible to calculate the course of the wavy line.
A third diagram, modified from that of Meyer, has been constructed by Professor Orme Masson, of Melbourne. The chief difference is that instead of grouping elements of the iron, palladium and platinum groups, they are distributed; hydrogen forms the first element of the fluorine column; and the long and short periods are kept separate by a fold in the diagram. A diagonal line, too, divides the 'metallic' from the 'non-metallic' elements.
Other devices have been suggested in order to represent diagrammatically the relations between the atomic weights; but it must be borne in mind that whatever system is employed, such plans are merely aids to thought, and have no real significance. They are on a par with the representation of numerical relations as curves, and can convey nothing which is not already contained in the actual numbers.
It was Mendeléef who first drew attention to the progressive alteration of the valency of the elements in passing from left to right along the table. While the metals of the alkalies, lithium, sodium, potassium, rubidium and cæsium, are all monads, in as much as one atom of any one of these elements is able to replace one atom of hydrogen, the typical monad, elements of the beryllium group are dyads; hence while the formula of sodium chloride is NaCl, that of calcium chloride is CaCl2, that of boron chloride, BCl3, for boron is a triad; the chloride of the tetrad, carbon, CCl4, and so on. And considering the compounds with hydrogen, where these exist, we have BH3, corresponding to the chloride; CH4, NH3, OH2, and finally ClH and FH. As the valency alters by unity in each ease, it appeared reasonable to place the elements on the table in equidistant columns; or, as in Dr. Stone/s diagram, on equidistant lines, dividing the spiral curve into eight equal segments.
Meyer, however, showed that if the elements be mapped on square
paper, so that the vertical divisions correspond to the volumes occupied by unit weight of the solid or liquid elements, while the horizontal divisions correspond with the atomic weights, a certain amount of regularity is to be noticed, as is to be seen in the accompanying diagram. There, it will be noticed, the elements, sodium, potassium, rubidium and cesium, occur at the summits of somewhat irregular curves. Such relations, among others, led him to formulate the proposition that the properties of the elements are periodic functions of their atomic weights; that is, they vary in a systematic manner, either positively or negatively, as the scale of the atomic weights is ascended.
The division of the elements into metals and non-metals corresponds broadly with another well-marked division—that into basic and acidic. Generally speaking, it is the oxides of the metallic elements which react with water to form bases; and those of the non-metals which form acids with water. This distinction was recognized by Lavoisier, when he named oxygen the acid-forming element. But what is a base? And what is an acid? The old definition was—two classes of substances, which, when brought together, react to form a salt. But the definition may now be made with greater definiteness. It was known to Cavendish and to Priestley, that when a current of electricity was passed through the solution of a salt in water, one portion of the salt, namely the basic portion, came towards the negative pole; and it was believed that this was due to the basic portion possessing a positive charge. Similarly, the acid portion, possessing a negative charge, traveled towards the positive pole. Hence, bases were said to be electro-negative, and acids, electro-positive. And Sir Humphry Davy arranged the elements m a series, of which one was supposed to be electro-positive to its neighbor on the right, and electro-negative to its left-hand neighbor.
According to modern ideas, bases, by the mere act of solution in water, are supposed to be split up into two portions, for which the term ion, invented by Faraday, has been retained; one ion is charged by the process of solution with a positive charge, and that portion is usually a metal; the other portion, which consists of one or more groups of hydrogen and oxygen in combination, termed 'hydroxyl'—OH—has a negative charge. A base, indeed, is a compound which splits in this manner. On the other hand, an acid, when dissolved in water, undergoes an analogous split; but in this case the electro-positive ion is always hydrogen, while the electro-negative ion may either be an element such as chlorine, or a group of elements such as exist in nitric acid (NO3).
The order of the various elements in the electric series has been determined; and not merely determined, but to each has been attached a numerical value. This value is identical with what is termed 'chemical affinity'; and it represents the electric potential of the element with reference to an arbitrary starting-point, which does not differ much from that of nickel, an element closely related to iron. Only a few such values have as yet been determined numerically; instances may be chosen from the magnesium group, where the numbers run: Magnesium 1.2; Zinc 0.5; Cadmium 0.19; or from the fluorine column, where the numbers are: Fluorine 2.0; Chlorine 1.6; Iodine 0.4. In each case the potential, positive or negative, is the highest for the element with smallest atomic weight, and decreases with increase of atomic weight, for elements in the same column. The order of some of the elements is: Cs Rb K Na Li Ba Sr Ca Mg Al Mn Zn Cd Fe″ Co Ni Pb H Cu Ag Hg′ Pt‴ ′ Au′ ″; and for electro-negative ions, S″ O″ I Br CI F; the first element, cæsium, being the most electro-positive, and the last, fluorine, the most electro-negative.
The order given above corresponds fairly well with the order in the periodic table, passing from left to right. But, as in the table, the atomic weights follow each other continuously round the cylinder or round the spiral, the abrupt change from elements of an extreme electronegative character, like fluorine to sodium, an element of highly electropositive character, or from chlorine to potassium, has always appeared remarkable. The old dictum, Natura nihil fit per saltum, if not always true (else we should have no elements at all, but a gradual and continuous transition from one kind of matter to another—a condition of affairs hardly possible to realize), has generally some spice of truth in it; and it might have been predicted (and the forecast seems to have been made obscurely by several speculators) that a series of elements should exist which should exhibit no electric polarity whatever. Such elements, too, should form no compounds, and, of course, should display no valency; they should be indifferent, inactive bodies, with no chemical properties.
The discovery of argon in 1894, followed by that of terrestrial helium in 1895, and of neon, krypton and xenon in 1898, has shown the justice of the foregoing remarks. In as much as the methods employed for the isolation of these elements illustrate their properties and confirm the views as to their inertness and lack of electric polarity, I propose to sketch shortly the history of their discovery.
An accurate investigation of the density of atmospheric nitrogen and of nitrogen prepared from its compounds led Lord Rayleigh to inquire into the cause of the discrepancy, for the density of the nitrogen of the atmosphere was found to exceed that of 'chemical nitrogen' by about one part in two hundred, whereas the accuracy of his experiments was such that it would have excluded an error of one part in five thousand. I need not here allude to the reasons which were at first put forward to account for this anomaly; suffice it to say that they offered no explanation; and that we ultimately traced the discrepancy to the presence in 'atmospheric nitrogen' of a gas nearly half as dense again as nitrogen. Two methods were adopted for isolating this gas. One was a repetition of a process which had been employed by Cavendish in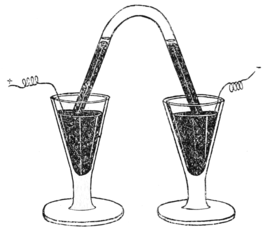 Fig. 1.1785; it consisted in passing electric sparks for a long time through air, confined over mercury, in presence of caustic alkali. The accompanying woodcut gives an idea of the apparatus he employed. This experiment had been made by Priestley ten years previously, but not with quantitative accuracy. It was Cavendish's object to inquire whether the nitrogen of the atmosphere had any claim to be regarded as a homogeneous substance, but he left the question undecided. Having continued to pass sparks through a measured volume of air, with the occasional addition of oxygen, until no
Fig. 1.1785; it consisted in passing electric sparks for a long time through air, confined over mercury, in presence of caustic alkali. The accompanying woodcut gives an idea of the apparatus he employed. This experiment had been made by Priestley ten years previously, but not with quantitative accuracy. It was Cavendish's object to inquire whether the nitrogen of the atmosphere had any claim to be regarded as a homogeneous substance, but he left the question undecided. Having continued to pass sparks through a measured volume of air, with the occasional addition of oxygen, until no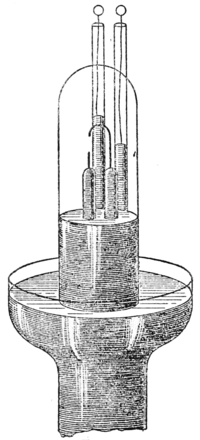 Fig. 2.further diminution in volume occurred, he found that after the excess of oxygen had been removed, the residue amounted to not more than 1/120th part of the whole of the nitrogen. The actual volume of the inactive gases in the nitrogen of the atmosphere is one eighty-fourth. Cavendish did not pursue the investigation further, and the discovery of argon was postponed for a century.
Fig. 2.further diminution in volume occurred, he found that after the excess of oxygen had been removed, the residue amounted to not more than 1/120th part of the whole of the nitrogen. The actual volume of the inactive gases in the nitrogen of the atmosphere is one eighty-fourth. Cavendish did not pursue the investigation further, and the discovery of argon was postponed for a century.
A convenient form of apparatus for repeating Cavendish's experiment is shown in the accompanying figure. The gas, air mixed with oxygen, is confined over mercury in an inverted test-tube, in contact with a few drops of a solution of caustic potash; and by connecting the rings with wires from the secondary coil of an induction apparatus, sparks pass between the platinum terminals in the interior of the test-tube. The volume of the gas rapidly diminishes; and in a few hours, the gas is removed to a clean tube, and the excess of oxygen absorbed by burning phosphorus; the inert gases remain behind. On a larger scale, the apparatus used by Lord Rayleigh, consisting of a balloon of six liters capacity, in the interior of which an electric flame is kept alight by means of a transformer, while a jet of caustic alkali forms a fountain in the interior, gives good results. By its help, seven or eight liters of mixed gases can be made to combine per hour.
Such experiments show the inactive nature of the argon group of gases towards an electro-negative element, oxygen. The gases are absolutely incombustible. No other elements can withstand such treatment, save platinum and its congeners, and gold. But even these metals combine with fluorine or chlorine, when heated in a current of one or other gas. Argon, however, is wholly unaffected when electric sparks are passed through its mixture with chlorine or fluorine, the two other most electro-negative elements. To them, too, it shows itself completely indifferent.
A more convenient method of separating the nitrogen from its admixture with argon in atmospheric air is by means of red-hot magnesium. The metal magnesium, Fig. 3.which is now made on a considerable scale for photographic and signaling purposes, is a white, silvery metal, which can be planed or turned into shavings. In the early experiments, a measured quantity of atmospheric nitrogen, dried by passing over suitable drying agents, was brought into contact with magnesium turnings, heated to redness in a tube of hard glass. It has been found, however, by M. Maquenne, that the metal calcium, which, for this purpose is most easily produced by heating together a mixture of magnesium filings and pure dry lime, is a more efficient absorbing agent for nitrogen, for it does not require such a high temperature, and can be effected without danger of melting the glass tube. Indeed, the operation is a very easy one, and can be carried out with the very simple apparatus shown in Figure 3. M. Guntz has also found that lithium, an element belonging to the same column in the periodic table as sodium and potassium, is an exceedingly good absorbent for nitrogen, for it tarnishes in nitrogen even at atmospheric temperature, owing to the formation of a nitride.
Fig. 3.which is now made on a considerable scale for photographic and signaling purposes, is a white, silvery metal, which can be planed or turned into shavings. In the early experiments, a measured quantity of atmospheric nitrogen, dried by passing over suitable drying agents, was brought into contact with magnesium turnings, heated to redness in a tube of hard glass. It has been found, however, by M. Maquenne, that the metal calcium, which, for this purpose is most easily produced by heating together a mixture of magnesium filings and pure dry lime, is a more efficient absorbing agent for nitrogen, for it does not require such a high temperature, and can be effected without danger of melting the glass tube. Indeed, the operation is a very easy one, and can be carried out with the very simple apparatus shown in Figure 3. M. Guntz has also found that lithium, an element belonging to the same column in the periodic table as sodium and potassium, is an exceedingly good absorbent for nitrogen, for it tarnishes in nitrogen even at atmospheric temperature, owing to the formation of a nitride.
On a large scale, the magnesium turnings are contained in iron tubes, and the gas-holders are made of copper or of galvanized iron. By this means, fifteen liters of argon were separated from about two cubic yards of air.
The inactivity of argon in contact with such highly electro-positive elements as lithium, magnesium and calcium again demonstrates its want of electric polarity. No other elements would have resisted such treatment, except those of the argon group. But these are not the only data from which such a conclusion can be drawn; for it was found that no action takes place between argon and hydrogen, phosphorus, sulphur, tellurium, caustic soda, potassium nitrate, sodium peroxide, sodium persulphide, nitro-hydrochloric acid, bromine-water and many other reagents which it would be tedious to mention, all of which are remarkable for their chemical activity. We may therefore take it that the name 'argon,' which means 'inactive,' has been happily chosen.
In attempting to form compounds of argon, however, another consideration was not lost sight of; if compounds of argon were capable of existence, they ought to exist in nature; and as in all probability they would be easily decomposed by heat, it ought to be possible to decompose them with evolution of argon, which could be collected and tested. Professor Miers, in a letter which he wrote me the day after an account of the fruitless attempts to cause argon to combine had been given to the Royal Society, drew my attention to experiments by Dr. Hillebrand of the United States Geological Survey, in course of which he obtained a gas, which he believed to be nitrogen, by treating the rare mineral clevite, a substance found in felspathic rocks in the south of Norway, with sulphuric acid. The chief constituents of clevite are oxides of the rare elements uranium and thorium, and of lead. The gas obtained thus, after purification from nitrogen, was examined in a Plücker tube with the spectroscope, and exhibited a number of brilliant lines, of which the most remarkable was one in the yellow part of the spectrum, similar in color to the light given out by the glowing tube. The position of this line and of others which accompany it established the identity of this gas, not with argon, as was hoped, but with a supposed constituent of the sun's chromosphere, first observed by M. Janssen of Paris, during an eclipse which was visible in India in 1868. The late Sir Edward Frankland, and Sir Norman Lockyer, who studied the spectrum of the chromosphere, gave to the supposititious element, which they regarded as the cause of these lines, the name 'helium,' a word derived from ′ἤλιος′ Greek for 'the sun.' Having been placed on the track, I examined, with the assistance of Dr. Collie and Dr. Travers, no fewer than 51 minerals; while Sir Norman Lockyer examined 46 additional ones, which we had not examined; and in 19 minerals, almost all of them containing uranium, helium was found. Only one gave an argon spectrum, namely malacon. We also sought for argon and helium in meteorites, which all give off gas on heating; but in only one specimen, a meteorite from Augusta County, Virginia, was helium found, in this case accompanied by argon. All natural waters contain argon, for that gas is somewhat soluble in water (4.1 volumes per 100 of water at 15° C.); but some also contain helium, as for instance the gas from the Bath springs, which Lord Rayleigh found to contain argon mixed with about 8 per cent, of its volume of helium; and helium has also been found in mineral springs at Wildbad, and at Cauterets, in the Pyrenees. It would appear, then, that helium is not such a very rare constituent of our globe; and indeed, it is probable that it is continually escaping from the earth in small quantities in certain regions.
Let us next turn our attention to the atomic weights of these elements, in order to discover what position should be assigned to them in the periodic table. It is not difficult to ascertain their molecular weights; that is the relative weights of equal numbers of molecules; for, assuming Avogadro's hypothesis, that equal volumes of gases contain equal numbers of molecules, or particles capable of independent existence in space, the weights of equal volumes of these gases, compared with that of an equal volume of oxygen taken as 16 (the usual standard) will give the relative weights of their molecules. The density of helium was found to be very nearly 2, or one eighth of that of oxygen; while that of argon was 19.94, very nearly 20. It may be interesting to spend a few minutes in a description of the method by which the density is determined. The principle is to weigh a globe, completely emptied of air by means of an air-pump; the globe is then filled with the gas, care being taken to observe accurately the temperature and pressure of the atmosphere at the moment of closing the globe; and the difference in weight of the full and the empty bulb gives the weight of a known volume of the gas. It is easy to compare it with that of an equal volume of oxygen.
The weight of a gas is much more considerable than might be supposed. Thus, a liter of oxygen weighs nearly a gram and a half; and the air in an ordinary room twelve feet broad, long and high, weighs over a hundred and fifty pounds. It is possible to obtain fair results by weighing as little as 30 cubic centimeters, or about one fluid ounce of any gas. Such a globe filled with helium weighs about one two-hundredths of a gram; and it is not difficult to be fairly certain to one-hundredth part of that weight. With a heavier gas like argon, much greater accuracy is of course possible.
Now, although the standard atomic weight with which others are compared, that of oxygen, is taken as 16, it is believed, for reasons which will afterwards appear, that a molecule of oxygen consists of two atoms, the weight of which will of course be 32. And, as the weight of helium is one eighth of that of an equal volume of oxygen, the weight of a molecule of helium will be the eighth part of 32, or 4. Similarly, the molecular weight of argon compared with that of an atom of oxygen taken as 16 will be 40, But the question has still to be answered: Does a molecule of helium or of argon resemble a molecule of oxygen in consisting of two atoms; or does it consist of one atom or of more than two?
It is believed that when heat is put into a gas, it is expended in causing the molecules to move. This motion may in some cases be of two kinds; the molecules may be urged through space, each molecule traveling in a straight path, until a collision takes place with another molecule, when it changes its rate and direction of motion; such motion i? termed 'translational motion.' On the other hand, if the molecules are themselves complex, that is, if they consist of groups of atoms, any energy imparted to the gas in the form of heat will produce, not merely the translational motion, but will also cause the atoms to move relatively to each other within the molecule. It is only the translational motion which is manifested as pressure, for it is only by their motion through space that the molecules can bombard the sides of the vessel which contains them, and so exert pressure on the walls. Hence it will require a less amount of heat to raise pressure in a gas with simple molecules, than in one of which the molecules are complex, for in the former case no heat is used in causing internal motion. Now, to measure such quantities of heat is by no means easy, although it has been successfully accomplished in some instances. To avoid this measurement, a device is adopted which produces equally satisfactory results. It consists in comparing the amounts of heat required to raise the temperature of a gas, first, when it is not allowed to expand, and when all the heat is used in producing molecular motion of the kind referred to; and second, when it is allowed to expand, and consequently when it could be made to do work; for example, to drive a small air-engine. In the latter case, a greater amount of heat is required to rise the temperature of the gas; an amount equivalent to the work which the gas does on expanding. This quantity, however, which is equivalent to mechanical work, is the same for all gases, provided equal numbers of molecules (or equal volumes) be heated through the same number of degrees of temperature. And this renders it possible to calculate the amount of heat required to raise the temperature of a gas, even without a direct measurement. An example will serve to render this somewhat difficult conception clear. For mercury-gas, for argon and for helium, and indeed for all gases, nitrogen, oxygen and their mixture, air, if a volume which contains the molecular weight of the gas taken in grams be raised through one degree of temperature, allowing the gas to expand, and so to do work, the amount of heat equivalent to this work is sufficient to raise the temperature of two grams of water through one degree. This is termed in the language of heat-measurement 2 calories. Now, the total heat required to raise the temperature of 40 grams of argon, for example (and it must be remembered that 40 is the molecular weight of argon), through 1°, allowing it to expand while it is being heated, is 5 calories. Deducting the 2 calories required for external work, 3 calories remain as the heat required to raise the temperature of the gas. For oxygen, on the other hand, the heat required to raise the temperature of 32 grams, its molecular weight, through 1° is 7 calories; and deducting 2 as before, the remainder is 5, the specific heat of oxygen. Hence for argon and for oxygen, we have the properties:
Heat Required.
| No external work. | External work. | ||||||
| Argon | 3 | : | 5 | :: | 1 | : | 1 23 |
| Oxygen | 5 | : | 7 | :: | 1 | : | 1 25 |
The argument stands thus: The heat required to raise the temperature of argon without expansion can be accounted for entirely on the supposition that it is wholly used in causing the molecules to move through space; on the other hand, more heat requires to be communicated to oxygen than to argon in the proportion of 3 to 5. With oxygen and similar gases, this extra heat must be doing something; it is supposed to produce motion of the atoms within the molecule. There is no such motion within the argon molecule; hence it is concluded that the molecule consists of a single atom; and in. that case, the molecular weight is the same as the atomic weight. The molecule of oxygen may be considered as possessing a structure like that of a dumb-bell; the atoms forming the knobs at each end of the bar. On throwing a dumb-bell through space, it will not merely change its position as a whole; but it will rotate. But a molecule of argon or helium is imagined to have the simpler form of a sphere or ball; when it is thrown practically no energy is used in causing it to rotate, but it is all expended in making it pass through space.
I must apologize for introducing such abstruse conceptions into a popular exposition; but they are necessary to the argument; and I am afraid that no simpler means can be found of reaching the conclusion that the molecules of argon and of helium are identical with their atoms.
As 4 is the molecular weight of helium, and as 40 is that of argon, these numbers also stand for their atomic weights. Let us next see how these figures fit into the periodic table.
In 1897, as president of the Chemical Section of the British Association, I chose the title An Undiscovered Gas' for the address to the Section. The arguments in favor of the existence of such a gas were briefly these: The differences between the atomic weights of consecutive elements in the columns of the periodic table are approximately 16 to 20; thus 16.5 is the difference between the atomic weights of fluorine and chlorine; 16, between those of oxygen and sulphur, and so on. Again, stepping one pace down the scale, we have 19.5 as the difference between chlorine and manganese; 20.3, between sulphur and chromium; 19.8, between silicon and titanium, etc. The total difference between manganese and fluorine is 36; between chromium and oxygen, 36.3; between vanadium and nitrogen, 37.4, and between titanium and carbon, 36.1. This is approximately the difference between the atomic weights of helium and argon, 36. I quote now from that address: "There should, therefore, be an undiscovered element between helium and argon, with an atomic weight 16 units higher than that of helium, and 20 units lower than that of argon, namely 20. And if this unknown element, like helium and argon, should prove to consist of monatomic molecules, then its density should be half its atomic weight, 10. And pushing the analogy still further, it is to be expected that this element should be as indifferent to union with other elements as the two allied elements."
Those who care to read the story of the search for this undiscovered element may find it in the address. Minerals from all parts of the globe, mineral waters from Britain, France and Iceland, meteorites from interstellar space; all these were investigated without result. Helium from various minerals was separated by long and tedious processes of diffusion into a possibly lighter portion, diffusing more rapidly, and a possibly heavier portion, diffusing more slowly; but with no positive result. The systematic diffusion of argon, however, gave a faint indication of where to seek for the missing element, for the density of the more rapidly diffusing portion was 19.93, while that of the portion which diffused more slowly was 20.01.
The invention by Dr. Hampson of an apparatus by means of which it is possible to obtain liquid air at small expense and with little trouble placed a new instrument in our hands; and Dr. Travers and I prepared 15 liters of argon from the atmosphere, with the purpose of distilling it fractionally, after liquefaction; for we knew, from the researches of Professor Olszewski of Cracow, who has done so much to determine the properties of liquefied gases, that argon could be liquefied easily by compressing it into a vessel cooled by help of liquid air. And, moreover, we were in hope that by fractionating the air itself, gases of even higher atomic weight than argon might possibly be obtained. Both expectations were realized; on distilling liquid argon, the first portions of gas to boil off were found to be lighter than argon; and on allowing liquid air to boil slowly away, heavier gases came off at the last. It was easy to recognize these gases by help of the spectroscope; for the light gas, to which we gave the name, neon, or 'the new one,' when electrically excited emits a brilliant flame-colored light; and one of the heavy gases, which we called krypton, or 'the hidden one,' is characterized by two brilliant lines, one in the yellow and one in the green part of the spectrum. The third gas, named xenon, or 'the stranger' gives out a greenish-blue light, and is remarkable for a very complex spectrum, in which blue lines are conspicuous.
Although neon was first obtained by the fractional distillation of argon, it was afterwards found convenient to prepare it direct from air. The torpedo-compressor, which is used for compressing the air before it enters Dr. Hampson's liquefier, was made to take in the air which had escaped liquefaction in the liquefier; the denser portions were thus liquefied, and the lighter portions were liquefied by compressing them into a vessel cooled by the denser fractions, boiling under reduced pressure, and consequently at a specially low temperature. This liquefied portion was again fractionated, and yielded neon; and it was not long before we discovered that helium was also present in the mixture. The presence of helium in atmospheric air had previously been noted by Professor Kayser of Bonn, and by Professor Friedländer of Berlin, on submitting the spectrum of argon to a searching examination.
The purification of this mixture of neon and helium from argon, although a lengthy process, was not attended by any special difficulty. It was accomplished by repeated distillation, the lighter portions being always collected separately from the heavier portions, and again distilled by themselves. But after this separation had been accomplished, we found that we were unable by means of liquid air to liquefy the mixture, or indeed any portion of it. We effected a partial separation by diffusion; but it is not possible to separate by this method two gases of which the quantity is limited. Another attempt was made by dissolving the gases in liquid oxygen, on the supposition that neon might prove more soluble than helium; but without satisfactory results. It was evident that a lower temperature than that possible by help of liquid air was necessary.
Professor Dewar had by that time succeeded in producing liquid hydrogen in quantity, and had indicated the principle, which is identical with that of Dr. Hampson's air-liquefier, although he has not published any detailed account of his apparatus. Dr. Travers undertook to investigate the subject; and after four unsuccessful trials, he made a liquefier, with the help of Mr. Holding, the laboratory mechanician, by means of which a hundred cubic centimeters of liquid hydrogen could be easily and cheaply produced. There was then no difficulty in effecting the separation of neon from helium; for, while neon is practically non-volatile, when cooled by liquid hydrogen, remaining in the state of solid or liquid, even that enormously low temperature is not sufficient to convert helium into a liquid. Hence the gaseous helium could be pumped away from the non-gaseous neon, and the latter was obtained in a pure state.
The residues obtained from the evaporation of about thirty liters of liquid air, after being freed from oxygen and nitrogen, were liquefied by help of liquid air, and fractionated from each other. The separation offered no special difficulty, but was long and tedious. It soon appeared that when most of the argon had been removed, the residue solidified when cooled; but while it was possible to remove the krypton by pumping, for it goes into gas slowly even at the low temperature of liquid air, very little xenon accompanied it; for at that temperature, xenon is hardly at all volatile.
Having finally separated the gases, their densities and other properties were carefully determined; and it was also proved that they are like argon and helium, in as much as their molecules consist of single atoms. Neon, as was expected, turned out to be the missing link between helium and argon; the atomic weight of krypton was found to be 81.6, and that of xenon, 128. The volumes occupied by equal numbers of molecules of the liquefied gases were determined; and also the boiling-points and melting-points of argon, krypton and xenon. These figures are shown in the following table:
| Helium. | Neon. | Argon. | Krypton. | Xenon. | |||||||
| Density of gas | 1 | .98 | 9 | .96 | 19 | .96 | 40 | .78 | 64 | .0 | |
| Atomic weight | 3 | .96 | 19 | .92 | 39 | .92 | 81 | .56 | 128 | .0 | |
| Density of liquid | 0 | .3(?) | 1 | .0(?) | 1 | .212 | 2 | .155 | 3 | .52 | |
| Boiling-points | 186 | .1°C. | -151 | .7°C. | -109 | .1°C. | |||||
| Melting-points | 187 | .9°C. | -169 | .°C. | -140 | .°C. | |||||
| Critical temperatures | 117 | .4°C. | -62 | .5°C. | +14 | .75°C. | |||||
| Critical pressures | (Metres.) | 40 | .20 | 41 | .24 | 43 | .50 | ||||
| Refractivity of gas | 0 | .124 | 0 | .235 | 0 | .968 | 1 | .450 | 2 | .368 | |
In every case there is seen what is termed periodicity; that is, a gradual alteration with rise of atomic weight, of the densities of the liquids, of the melting-points, of the boiling-points, and of the retardation of light when passed through the gas.
Let us consider, in conclusion, the position of these elements in the periodic table; and it will be sufficient to confine our attention to the groups of elements which form the neighboring columns. The atomic weights are given in round numbers.
| Hydrogen. | Helium. | Lithium. | Beryllium. |
| 1 | 4 | 7 | 9 |
| Fluorine. | Neon. | Sodium. | Magnesium. |
| 19 | 20 | 23 | 24 |
| Chlorine. | Argon. | Potassium. | Calcium. |
| 35.5 | 40 | 39 | 40 |
| Bromine. | Krypton. | Rubidium. | Strontium. |
| 80 | 82 | 85 | 87 |
| Iodine. | Xenon. | Cæsium. | Barium. |
| 127 | 128 | 133 | 137 |
It is evident that these new elements fall into their natural places between the strongly electro-negative elements of the fluorine group, and the very electro-positive elements of the lithium group; and that, in consequence of their lack of electric polarity, and their inactivity, they form, in a certain sense, a connecting link between the two. It is curious, too, to notice that iodine, xenon, cæsium and barium form the ends of their respective columns. It is, of course, not impossible that other elements may be discovered, possessing similar properties, and yet higher atomic weights than these; but as yet there is no clue to guide us where to search for them.
It is difficult, owing to the impossibility of effecting a complete separation of the inactive elements from each other, to do more than hazard a guess as to their relative amount in air. As they are easily separated from the other constituents of air, there is no doubt as to their total amount; air contains 0.937 parts of argon and its companions by volume in 100 parts. Perhaps the table below may be taken as affording some indication of their relative amounts. Air contains by volume:
0.937 parts of argon per hundred.
One or two parts of neon per hundred thousand.
One or two parts of helium per million.
About one part of krypton per million.
About one part of xenon per twenty million.
It is of course not impossible that xenon may contain an even smaller proportion of a still heavier gas; but it is unlikely. Sea-water sometimes contains a grain of gold per ton; that is one part in 15,180,000; a grain of xenon is contained in about four hundredweights of air.
The problems suggested by the periodic table are by no means solved by the discovery of these aerial gases; but something has been done to throw light upon one obscure comer of the field. The gap between the electro-positive and the electro-negative elements has been bridged.


